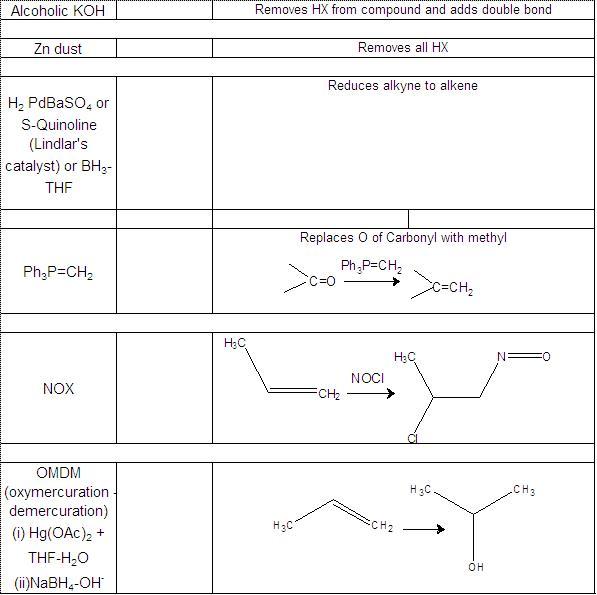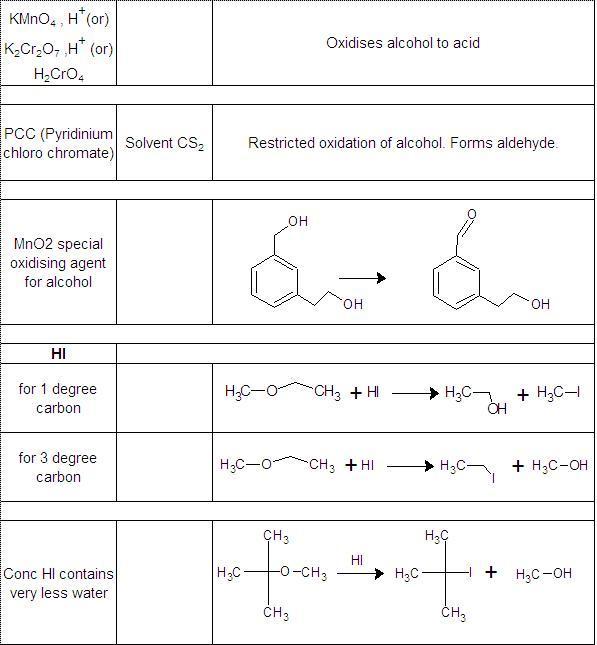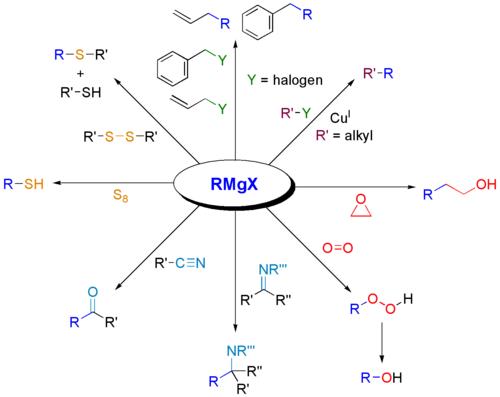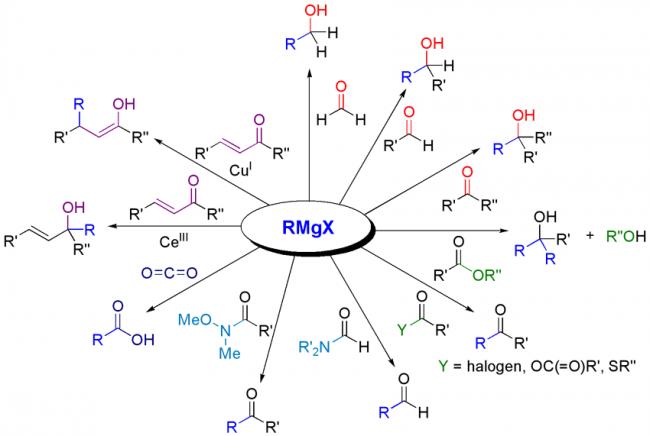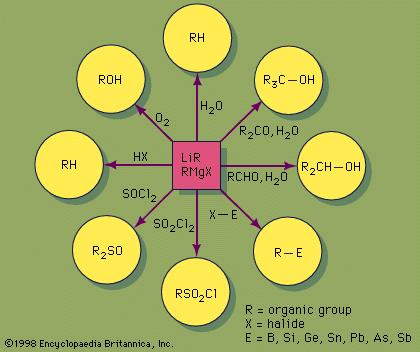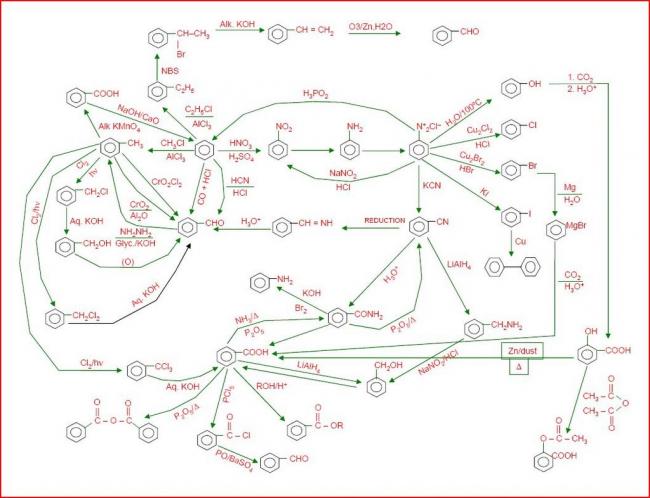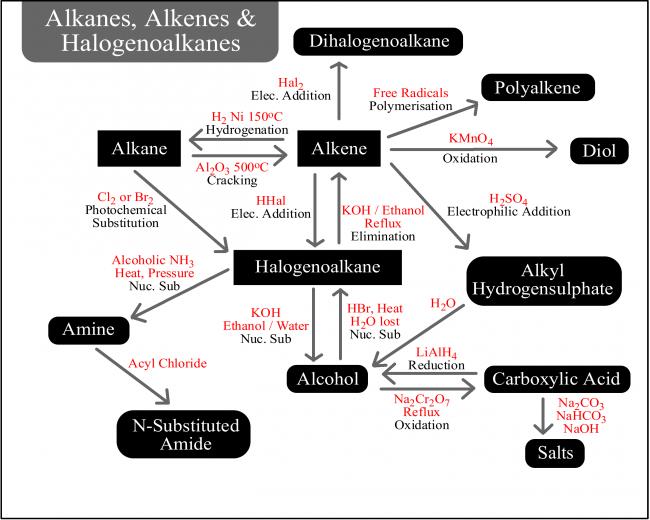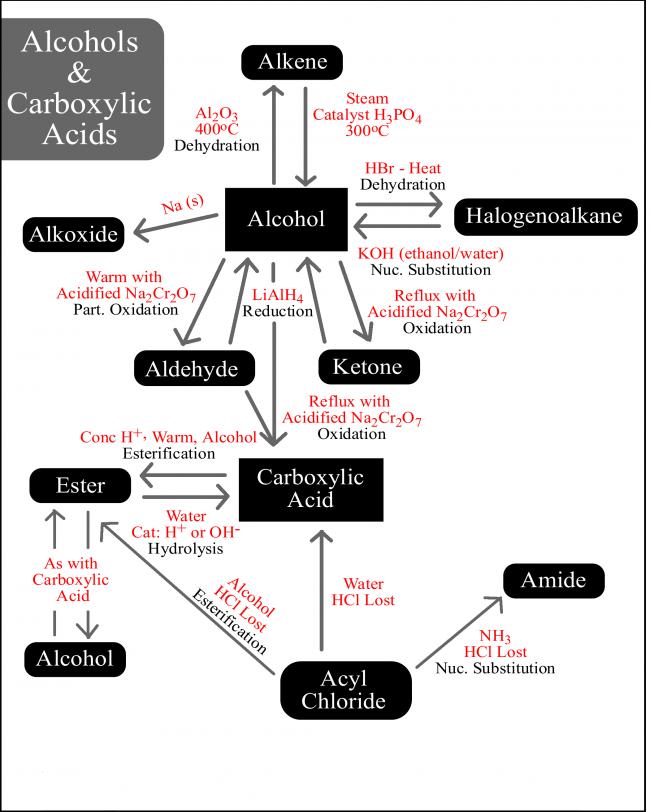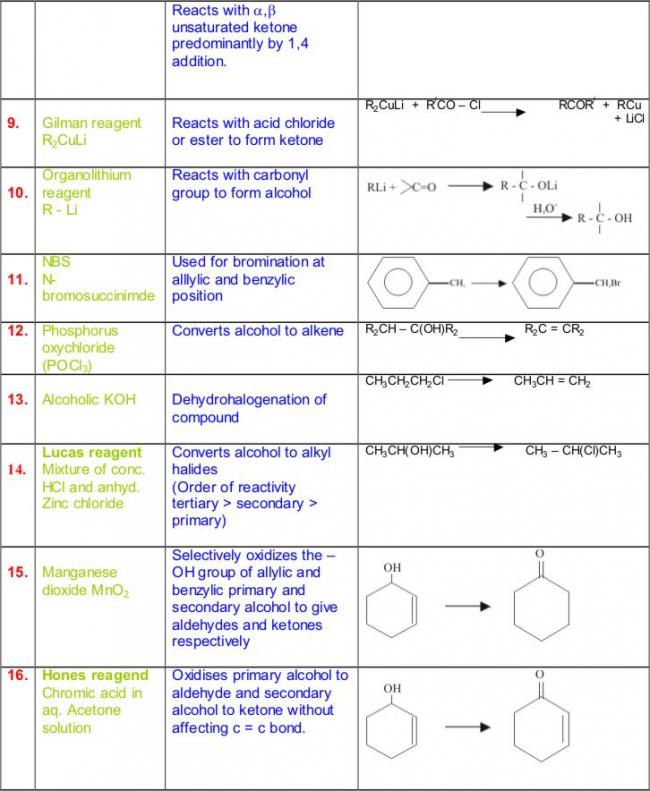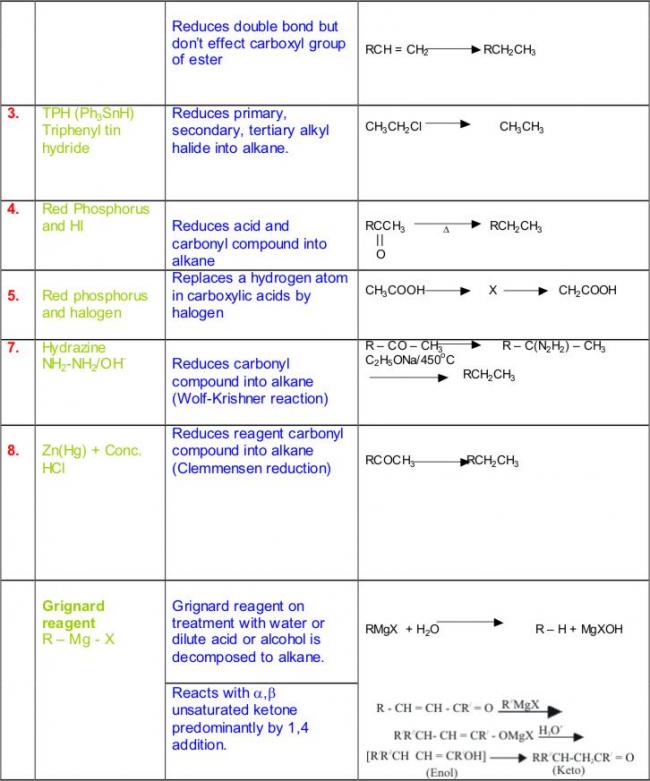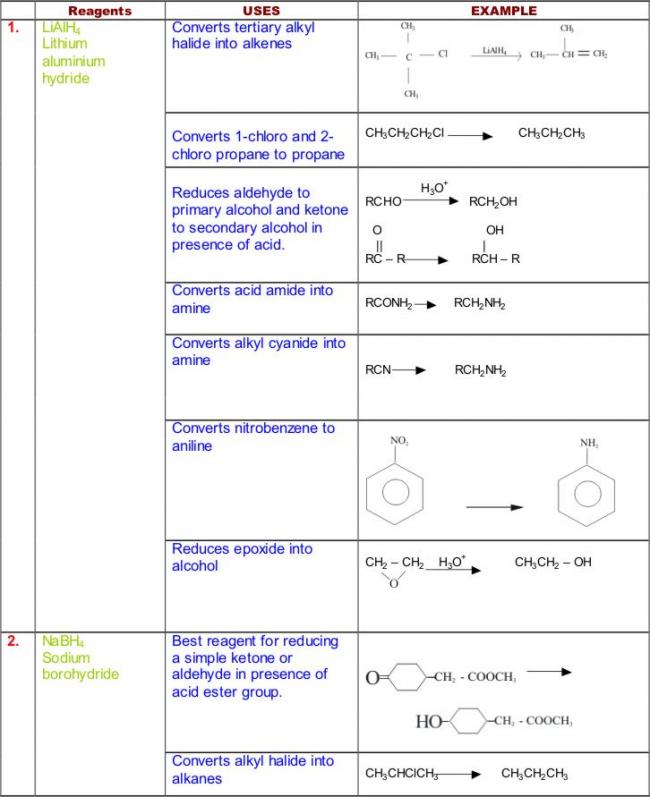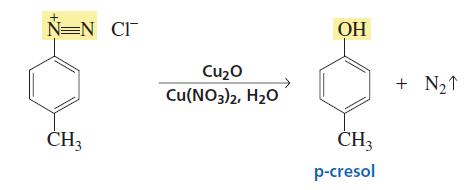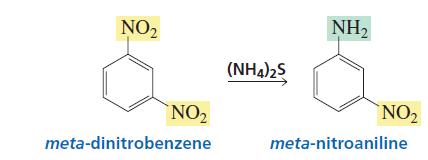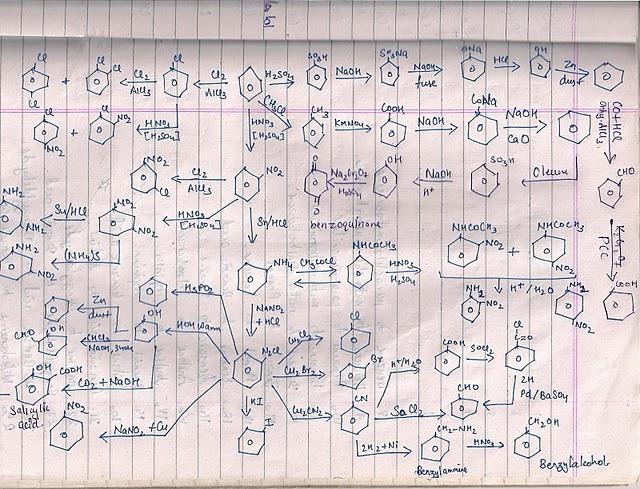
Not to be outdone by Manmay's and Anand's excellent threads, I am making a thread for organic reagents. Post as many as you can! I begin with the chapter alkyl halides. You may continue it or post the functions of other reagents you know.
1. Aqueous KOH - SN2, OH-
2. Aq KCN - SN2, CN-
3. AgCN - SN1(If the leaving group is halide, and nitrogen being a hard base combines with the hard acid carbocation formed, as per HASB theory, since this is ambident nucleophile), otherwise SN2 (here it will be carbon attacking, being a soft base attacking a soft acid partially charged carbon)
4. KSH - SN2, SH-
5. KNO2 - SN2, ONO-
6. AgNO2 - SN1, NO2-
7. Moist Ag2O => 2AgOH, SN2, OH-
8. Dry Ag2O => Ether formation by SN2
9. R-X + NH3 -> R-NH2
RNH2 + Excess R-X ---> R2NH
R2NH + Excess R-X ---> R3N
R3N + Excess R-X ---> R4N+X- (Tetraalkylamine salt)
-
UP 0 DOWN 0 6 24

24 Answers
1.FOR ALKOXY MERCURATION
Hg(OCOCF3)2,ROH,NaBH4
2.FREMY'S SALT (K2[NO(SO3)2])
3.CuO.CuCr2o4(FOR REDUCTION OF ESTERS TO ALCOHOL)
i found this under arene oxides.....
1. cytochrome,P450
benzene------------------------------> benzene oxide
2. diethyl ether or THF -----
The solvent (usually diethyl ether or tetrahydrofuran) plays a crucial role in the
formation of a Grignard reagent. Because the magnesium atom of a Grignard reagent is
surrounded by only four electrons, it needs two more pairs of electrons to form an octet.
Solvent molecules provide these electrons by coordinating (supplying electron pairs) to
the metal. Coordination allows the Grignard reagent to dissolve in the solvent and
prevents it from coating the magnesium shavings, which would make them unreactive.
1 more thing wch i nid 2 mention......... i hav copied dis 4rm sumwhere .....long ago........ i dunt xactly remebr da source now...... thought wud b useful ........so posted here :)
6) Gilmann's reagent
The first organometallic compounds used in coupling reactions were Gilman
reagents, also called organocuprates. They are prepared by the reaction of an organolithium
reagent with cuprous iodide in diethyl ether or THF.
gilmann's reagent -- (CH3)2CuLi
Gilman reagents are very useful to synthetic chemists. When a Gilman reagent
reacts with an alkyl halide (with the exception of alkyl fluorides, which do not
undergo this reaction), one of the alkyl groups of the Gilman reagent replaces the
halogen. Notice that this means that an alkane can be formed from two alkyl
halides—one alkyl halide is used to form the Gilman reagent, which then reacts with
the second alkyl halide. The precise mechanism of the reaction is unknown, but is
thought to involve radicals.
Gilman reagents can be used to prepare compounds that cannot be prepared by using
nucleophilic substitution reactions. Gilman reagents can even replace halogens in compounds that contain other functional groups.
3) Heck reaction
uses Pd(PPh3)4 and (C2H5)3N as reagent.
The Heck reaction couples an aryl, benzyl, or vinyl halide or triflate
with an alkene in a basic solution.
4) Stille reaction
also a coupling reaction uses Pd(PPh3)4 and THF
The Stille reaction couples an aryl, benzyl, or vinyl halide or triflate with a stannane.
Aryl and vinyl groups are coupled preferentially, but an alkyl group can be coupled if
a tetraalkylstannane is used.
5) Suzuki reaction
also a coupling reaction uses Pd(PPh3)4 and NaOH
The Suzuki reaction couples an aryl, benzyl, or vinyl halide with an organoborane in
a basic solution.
i know they might not be useful but still posted
name of the reagent - used in
1.ACETIC ANHYDRIDE - DEHYDRATION
2.ACETYL CHLORIDE - NITRATION OF ARYL AMINES
3.ALUMINIUM DIMETHYLAMIDE - TRANSAMINATION
4.ALUMINIUM IODIDE - BAYLIS-HILMAN REACTION
5.ALUMINIUM TRIFLATE - CYCLOISOMERISATION
6.ALUMINA - HYDROHALOENATION OF ALKENES AND ALKYNES
7.BARIUM MANGANATE - OXIDATION OF DIOLS