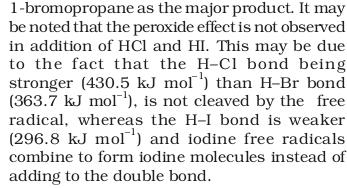
Source : NCERT class 11th
1..why Hcl and HI do not form radicals easily ?????????????
-
UP 0 DOWN 0 1 3

3 Answers
govind
·2010-03-14 23:15:36
Are u talking abt something related to Anti-Markonikov reaction...
in that case the formation of free radical is an exothermic phenomenon in case of HBr only....in case of HCl and HI it is an endothermic phenomenon..
Pritish Chakraborty
·2010-03-15 00:49:24
The bond dissociation enthalpy of H-Cl bond is too high to be broken simply by radical initiation or homolytically, and iodine free radicals are unstable (they combine to form I2 instead of continuing radical reaction). HBr beech ka hai isliye it works. HF will likely explode in your face if you try.