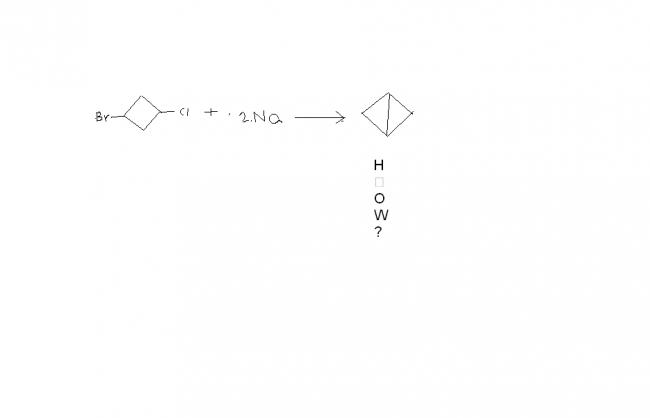
This question was given after the topic Wurtz reaction,,....so could it be related to Wurtz reaction??
Please don't add 'sayad' after your answer
4 Answers
It follows a radical anion mechanism.
First the bromine-carbon bond is homolytically broken to form a bromine radical and a carbon radical(rest of the compound).
One of the sodium atoms takes up this bromine atom. End of the radical story there.
Now the other sodium atom gives away an electron to the carbon compound radical and this sodium atom gets ionized to Na+. Now we have a radical which has been transformed to an anion.
Now SNi, or intramolecular substitution takes place as the end product will be two fused cyclopropane rings(formation of 3 membered ring is favoured by kinetics, but not by thermodynamics).
The chlorine atom thus pushed out is taken by Na+ to become NaCl.
Thus side products are NaBr and NaCl along with this product you have mentioned.
This sort of reaction is also called an intramolecular Wurtz reaction.