giv K2Cr2O7 and conc. H2SO4 it will oxidise methanol n form acetaldehyde then acetic acid.
CH3OH → CH3COOH
The above reaction proceeds in presence of..
(a) K2Cr2O7
(b)CH2N2
(c)CO2/BF3
(d) None
-
UP 0 DOWN 0 0 7

7 Answers
@nitu.. if you observe the reaction carefully, the acid has one carbon more. thus the reaction given by you will not answer the question.
I guess the answer to this question is d)None.
As the reaction is methyl carbonylation with takes place in the presence of a metal carbonyl, which is not present in any of the options.
@anirban is it correct?
Its not correct @ Somrwita
 Somrwita Mondal reason?????
Upvote·0· Reply ·2012-10-15 10:54:55
Somrwita Mondal reason?????
Upvote·0· Reply ·2012-10-15 10:54:55
The question is asking for one step reaction...i.e. directly acetic acid from methanol!
 Anirban Mukherjee And in that case what should be the reagent??
Anirban Mukherjee And in that case what should be the reagent??
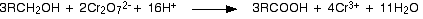
SOOO... K2Cr2O7 can directly convert alcohols to acids!!
 Subhojit Paul if you observe the reaction carefully, the acid has one carbon more. thus the reaction given by you will not answer the question.
Subhojit Paul if you observe the reaction carefully, the acid has one carbon more. thus the reaction given by you will not answer the question.
yes your answer is correct @ Subhojit..and the explanation is perfect too..:)