I THINK IT IS= 1>3>4>2
arrange according to stability order :
(i) CH3O C+H2
C+H2
(ii) 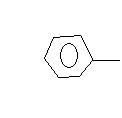 C+H2
C+H2
(iii) CH3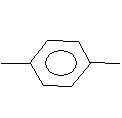 C+H2
C+H2
(iv) CH3C+H2
-
UP 0 DOWN 0 0 3

3 Answers
qwerty
·2010-09-29 19:03:42
1>3>2>4
delocalise the lone pair of oxygen towards the ring , and u see that carbocation is also in conjugation.
now in 2 also there is +M effect of Phenyl but in 1 the resonating structure has octet of all atoms complete hence is more stable.
thus 1 > 2
3 has +M of phenyl and 3 HC from methyl so 3> 2 , and same reason for 1>3 as for 1 > 2
4th has only hc rest all hav resonance.
so 4 < all
hence 1 > 3 >2 > 4