1)napthalene is two benzene rings attached together side by side the central two carbon to which they are attached experience high strain so there length gets reduced and so all do not have the same lengths
Briefly explain the following observations. Where possible and appropriate, use structures and/or mechanisms to support your answers.
(a) The carbon-carbon bonds in naphthalene are not equal in length.
(b) The resonance energy/ring for naphthalene is less than that for benzene.
(c) Attack occurs faster at C-1 in naphthalene than at C-2, but substitution at C-2 gives a more stable product.
(d) 2-naphthol undergoes electrophilic attack at C-1 but not at C-3.
(e) 2-naphthoic acid cannot be prepared by chromic acid oxidation of 2-methylnaphthalene. (What happens instead?).
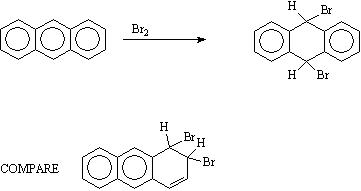
(f) Bromination of anthracene occurs in the middle ring rather than the two outer rings.
-
UP 0 DOWN 0 0 6

6 Answers
c) when attack takes place at C-1 there are more number of resonating structures of the intermediate than in the case of C-2
hence it is formed faster
but C-1 is sterically hindered by the hydrogen at the junction of the rings n no hindrance for C-2
so C-2 more stable
d) there are more resonating structures for C-2 than C-3
hence attack takes place at c-2 in 2-napthol
e) instead one of the ring gets oxidised
the ring with CH3 attached gets oxidised to form 1,2 benzene di carboxylic acid
(a)
A combination of the above structures to give the resonance hybrid gives unequal bond lengths.
(b)
The resonance structures for benzene are only benzenoid. Naphthalene also has contribution from quinanoid forms (see above) which are less stable than benzenoid.
(c) 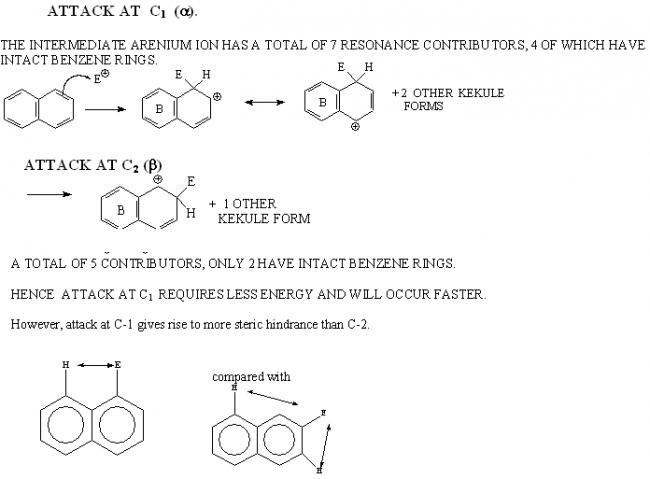
(d)

(e)
Chromic acid oxidation of 2-methylnaphthalene causes oxidation of the ring rather than the methyl group. The compound formed is :-
(f)
When attack occurs in the middle ring, two intact benzene rings remain giving a total of 2 x 36 = 72 kcal. resonance energy. If attack occurred in one of the two outer rings then a naphthalene ring comprising 61.5 kcal res. energy would remain.