yes
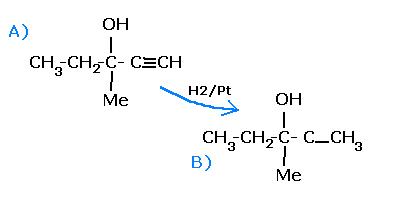
An optically active alcohol (A) [C6H10O] absorbs 2 moles of hydrogen per mole of [A] upon catalytic hydrogenation and gives a product [B].[B] is resistant to oxidation by CrO3 and does not show any optical activity.What is not true regarding A and B ?
A. [B] is optically active.
B. [A] can easily be oxidized.
C. [B] shows +ve iodoform test
D. [A] shows -ve iodoform test.
-
UP 0 DOWN 0 0 3

3 Answers
govind
·2010-04-06 12:25:10
Got this one by eliminating the options...
A is false as given in the question
B is false as according to the hints given it shud be a tertiary alcohol..
C is also false due to the reason given abv
D is true as tertairy alcohols cant be oxidised..
I hope i am correct..