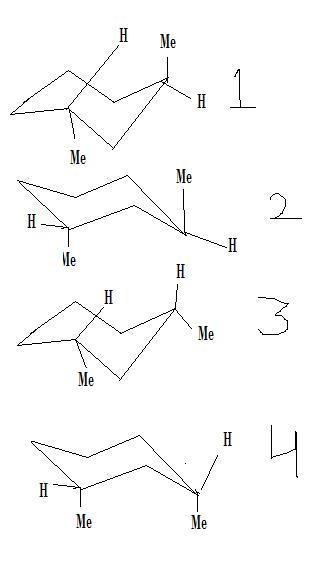
Since in ( 4 ) , the methyl groups are in immediate axial position , hence repulsion between these two groups in this conformer must be more than that taking place in any other possible conformer . This should lead to less stability of this conformer .
In ( 1 ) and ( 2 ) , the methyl groups are in the same axial position , but distant from each other . As the position of hydrogen atoms do not effect the stability of the conformer to a considerable extent , hence , these two must be of the same order of stability .
In ( 3 ) , the two methyl groups are furthest from each other , both being equatorial . Hence , its stability must be more than any other conformer .
So the required order : -
( 3 ) > ( 2 ) ≈ ( 1 ) > ( 4 )
As a general rule , always remember that the position of the two interacting methyl groups matters most when it comes to the stabilities of various conformers , i . e , depending on the various positions of the two methyl groups , we may safely say : -
Equatorial - Equatorial > Axial - Equatorial > Axial - Axial