 29
29Ans 5..is the answer 4?
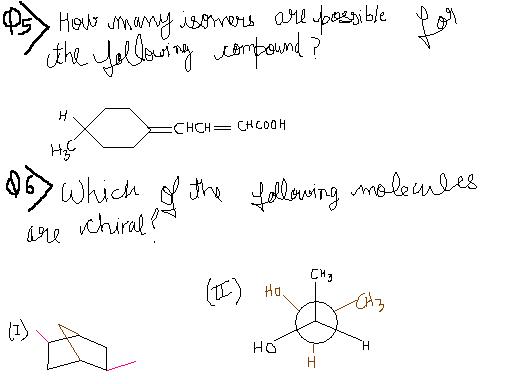
Ans 6 ...in the second compound rotation by 60° produces a meso compound..it has chiral centre but it's optically inactive due to internal compensation..the first compound has two chiral centres..
Ans 7..B
Ans 8.. where A reacts with reagent B ..i think it shud be R in place of B here..
 11
11@Govind, u r correct for ques 5,6,7
Ans 8) (b)
 29
29@Tushar..see my Ans 6.. sed both are chiral..but i have given additional info regarding that compound...and for Ques.8 ..no idea..
 11
11@ Govind, oh yeah sry ur 6th ans is right
Actually, I misunderstood the ques
For ques 5 , I am getiing 3 isomers. Where is my mistake ??
Wats ur reason for ans 7 ???
 29
29In question 5..two isomers bcoz of cis-trans..at the carbon attached to -COOH group..and the ctclic ring has one chiral centre..so 2*2 = 4..moreover i am not getting any kinda symmetry in this compound so ans remains 4 only...
ans 7..the method i used here was elimination...eclipsed to gausche needs energy abt 3Kcal/mol and for the changes in cyclohexane also needs less energy..so we have only one option remaining and that's B...
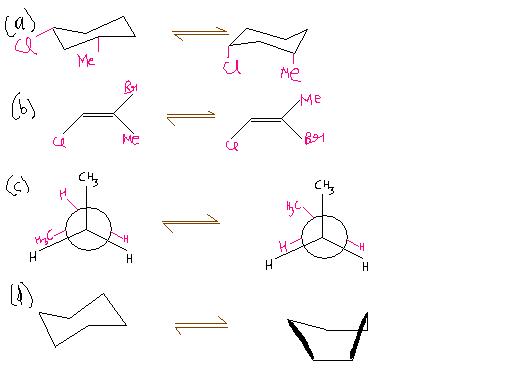
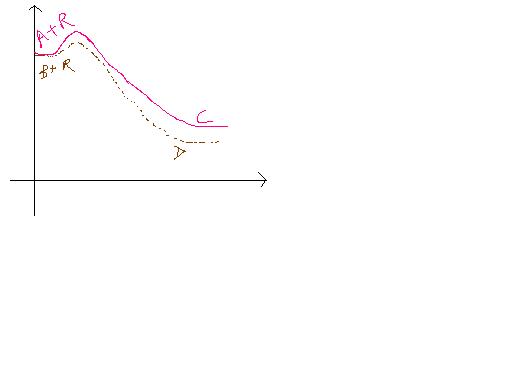 Consider the following energy profiles where A reacts with reagent B and gets converted to C, and B reacts with same reagent (R) under identical conditions and gets converted to D. Compounds A and B are enantiomers of each other. Which statements abt the T.S (Transition states) for these transformations must be true ?
Consider the following energy profiles where A reacts with reagent B and gets converted to C, and B reacts with same reagent (R) under identical conditions and gets converted to D. Compounds A and B are enantiomers of each other. Which statements abt the T.S (Transition states) for these transformations must be true ?