Carbon is pentavalent in b)...you sure the question is correct?
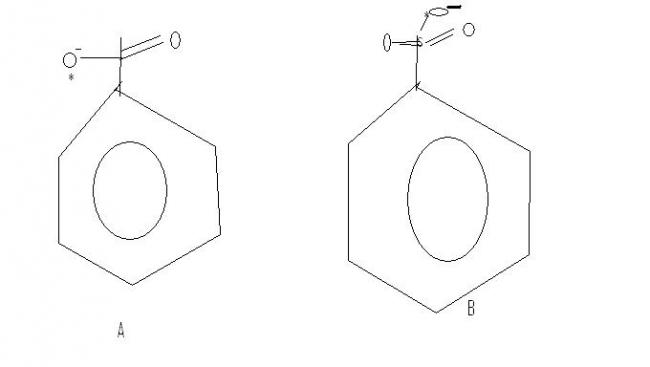
On what basis are the nucleophilic strengths being decided?
Which oxygen is more nucleophilic - A or B ?
-
UP 0 DOWN 0 0 7

7 Answers
You got an eagle eye..or you just saved the page and enlarged the image, asish..lol.
Then b) is the answer, more resonance, more delocalisation of negative charge on O, weaker basicity and stronger nucleophilicity...
pritish if u say that b is a good nucleophile than A then u mean that A is a better leaving grp than B , is it ? i dont think so
Leaving groups also are decided by weaker basicity...where am I going wrong, if I am? I wonder.
actually i didnt say make point clearly ,
see u said that weaker basicity implies stronger Nucleophilicity
so if its a good leaving grp then its a weak base and hence strong nucleophile as u said ,
but isnt it false?
But then resonance stabilised substrates having a negative charge are good nucleophiles..isn't it?
Or is that for target substrates which are 2 degree and above..