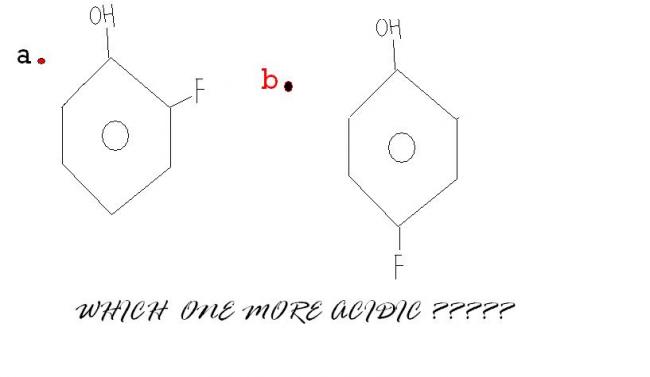
The second one is more acidic..coz in the first compound there will be intramolecular hydrogen bonding...
9 Answers
that's why i posted here.........
in the first compound there will be intramolecular hydrogen bonding.
but still order is given a>b [7]
Answer will be A
REASON :
Here we won't consider intramolecular H bonding in case B because - I effect is more
dominating over ortho effect {FACT}
If it were NO2 instead of F then ans wud be B
http://chemicalland21.com/lifescience/phar/o-FLUOROPHENOL.htm
http://chemicalland21.com/lifescience/phar/p-FLUOROPHENOL.htm
The inductive effect is more important here than the resonance effects because resonance structures where the fluorine has a + charge are very small contributors.
pKa of phenol = 10.0
pKa of ortho-fluorophenol = 8.81
pKa of meta-fluorophenol = 9.28
pKa of para-fluorophenol = 9.81
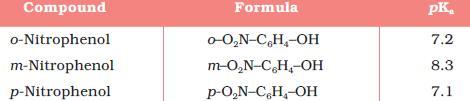
@govind- an analogy from above is NOT applicable, as F is more En than O
It is due to the -R effect of F.
As F is closer to OH in A therefore I'll gofor it!!!!