debotosh how is that helping in the formation of the 5 member ring????
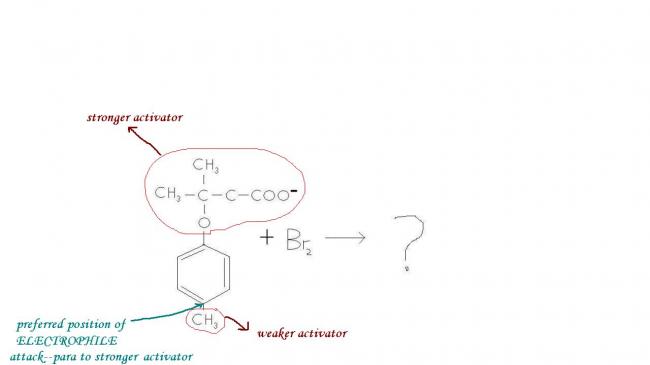
I came across a question wich was as follows :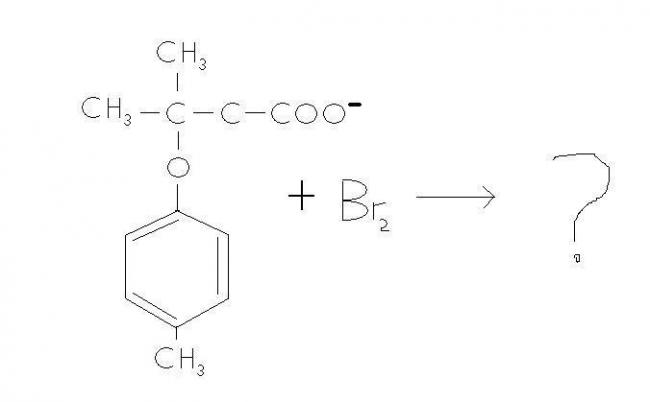
Now the product given in the ans is 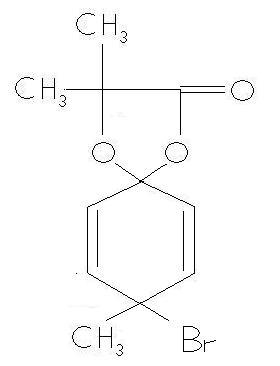
I agree that there is no problem in this answer
BUTTTTTTTTTTTTT wats wrong in the product that i m putting forward???? It has conjugation , and is a 6 membered ring !!!! where is the flaw ???? [2]
wat r ur views???
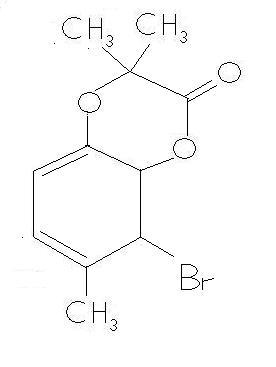
-
UP 0 DOWN 0 0 4

4 Answers
n i would say this is the case where the product is called abnormal product ...though it is not always abnormal....in riemer tiemenn reaction...
Following on from what debo said, if you go by the mechanism I think you will end up with a 5 membered ring...
After Br gets attached, aromaticity cannot be regained as the methyl carbocation is unstable, so base cannot abstract CH3+. The positive charge is shifted by resonance to the carbon atom holding the stronger activator group, from where COO- attacks the positive charge. O- is a hard base and a carbocation is a hard acid, so they join and make merry, forming a precipitate compound so that no further resonance occurs.
6 membered ring toh ban hi nahi sakta...just resonate the positive charge and you'll see what I mean.
Okay I'm uploading an image. The possibilities are 5 and 7 membered rings, and 5 membered ring is kinetically and thermodynamically favoured.