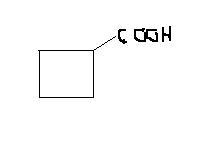
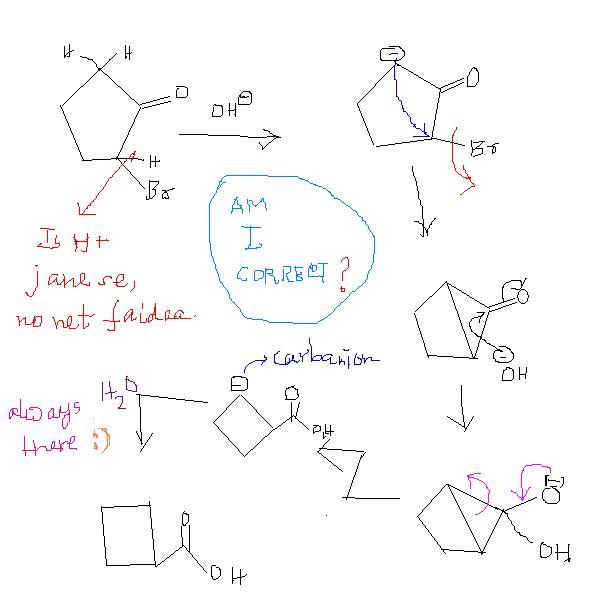
hint think of ring contraction in case of anions
2-bromocyclopentanone to cyclobutanecarboxylic acid. ( basic mdm)
-
UP 0 DOWN 0 0 9

9 Answers
skygirl
·2009-01-18 03:05:11
first, acid-base reaction.
then, H+ abstraction.
then, triangular ring formation.
then, attack on carbonyl carbon.
then, 3-memeber ring opens .. hence carbanion formation.
bus..