So finally we are stuck at what is rate of reaction :p
this is the beauty of chemistry.. I studied two years solved all kinetics questions and finally i came 2 know that i don't know what is the basic definition.
[3]
[2]
A reaction is taking place.. A is converting to B at certain rate..i.e.
A B
at t=0 10 0
t=2 mins 8 1
t=4 mins 6 2
...
..
Now if we write the reaction as
2A→B
then
rate=-d[A] = d[B]
2dt dt
rateavg(t=2,t=4)ΔA= 8-6 = 1/2
2Δt 2*2
------------------------------------------------------------------
but if we write reaction as A→1/2B
then
rate=-d[A] = 2d[B]
dt dt
then
rateavg(t=2,t=4)ΔA=8-6 = 1
Δt 2
so which rate is the rate of the reaction? [7]
Reply quickly.. totally confused [7]
Note:Earlier i was confused in this topic on this:
http://targetiit.com/iit_jee_forum/posts/kinetics_63_63_559.html
there i got some clarity in this concept but now i am stuck in this rate..
So finally we are stuck at what is rate of reaction :p
this is the beauty of chemistry.. I studied two years solved all kinetics questions and finally i came 2 know that i don't know what is the basic definition.
[3]
[2]
I'm telling that when we apply Δt→0 why are dividing by the stoichiometeric co-efficient?
Shouldn't 1/a d[A]/dt be just d[A]/dt?
There's no talk of stoichiometeric co-effs in this defenition[2]
yes and instant rate is defined as change in conc/time interval .. when time interval→0
inst rate=Lt (ΔA/Δt)
Δt→0
From what I read, avg rate was defined as the change in conc. of reactant divided by the time interval. Pls correct me if I'm wrong.
are whats the difference... when Δt→0 it becomes instantenous..
According to Jerrica IUPAC's Gold Book definition[1] the reaction rate v (also r or R) for a chemical reaction occurring in a closed system under constant-volume conditions, without a build-up of reaction intermediates, is defined as: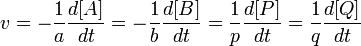
I was under the impression that the rate of disappearance of reactant is same as rate of rkn.
In between Manish bhaiya u said in the post i mentioned above ..
1st order reaction is one in which rate of disappearance of reactant is drectly prop to conc of reactant..
But every where (in NCERT) also it is given rate of rkn is prop to [A]
But if i follow this def then the problem continues as in above mentioned post..
but i follow ur def then only it gives correct results..
NCERT gives 1st def but uses 2nd def result in solving an example [7]
but i read somewhr dat the rate is dat for which one mole of product is prodused..
I m not sure of this but I think in this manner
In these cases if we have to find the rate of reaction, then the stoichiometric cofficients should be minimum possible integers.
so I one should give the answer
I don't think differential rate equation and average rate have any relation. Why did you use 1/2 in avg rate for I and 2 in avg rate in II? [7]
Does this question make any sense? Pls ignore it if it doesn't.
But if a reaction is taking place as shown in data
then we write the equation so what we will write.. it will affect the reaction rate which is taking place..
@Philip: Count me in as well, dude.
@ the pirate[3]:
Is the data authentic or are you just playing with numbers?[3]
I think Philip is right
We have to take both the reactions as different.
i think we take both reactions as different
decomposition of N2O5 also shows same conflict
dont take my word for it though.