1 Answers
Bitan Chakraborty
·2013-04-02 01:50:02
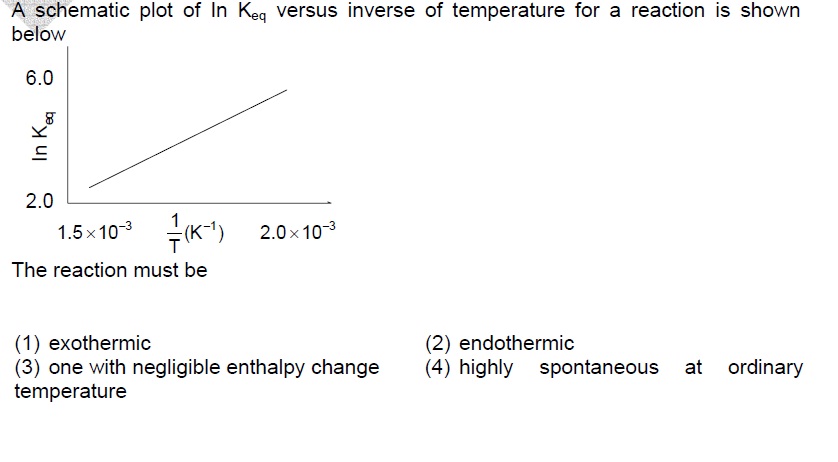
The van't Hoff equation provides information about the temperature dependence of the equilibrium constant.
∂(lnK)∂(1/T) = - dH0R
From the graph, we have the slope is +ve.
So, - dH0R > 0 => Heat is released during the reaction.
Thus, this is an exothermic reaction.
Answer: (1) exothermic.