(a) (b) (c) (d) -- agree with pritish
(e) 50 kJ absorbed
(f) 200 kJ
Q.1 : Answer the following questions based on the potential energy diagram shown here: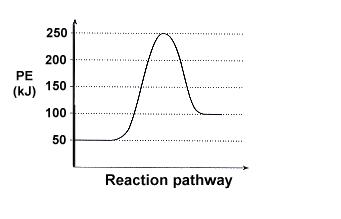
a. Does the graph represent an endothermic or exothermic reaction?
b. Label the position of the reactants, products, and activated complex.
c. Determine the heat of reaction, ΔH, (enthalpy change) for this reaction.
d. Determine the activation energy, Ea for this reaction.
e. How much energy is released or absorbed during the reaction?
f. How much energy is required for this reaction to occur?
a) The reaction profile represents an endothermic pathway as from a lower state of energy, further energy is absorbed to get to a higher state of energy as the energy hill shows.
b) Reactants at 50 kJ, activated complex at 250 kJ, products at 100 kJ.
c) 50 kJ?
d) 200 kJ
e) 150 kJ
(a) (b) (c) (d) -- agree with pritish
(e) 50 kJ absorbed
(f) 200 kJ