1.http://targetiit.com/iit-jee-forum/posts/just-a-second-13938.html
10 Answers
2) Strange!,,, why dosen't it form an inner-orb. complex when it is getting d2sp3 easily ?
4) Oxid.n No. =0, EAN= 24+2*(6)=36
5) [Mn(CO)6]+ > Cr(CO)6 > [V(CO)6]- ?
6) (d) ?
7) Most probably, (d) & (h) true.
8)- b,c (dunno about (a)).
9) Abhishek bhai,mein "Pt" ka Oxid.n state kya likhenege.....+2 or +4 ?
10) +2.
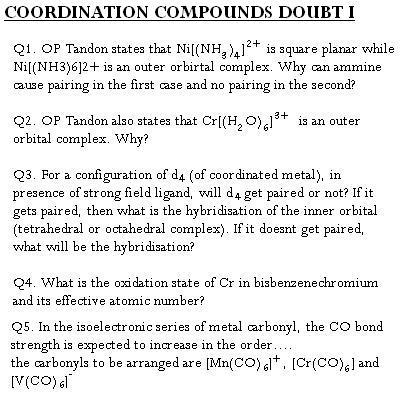
Ans 1..the satement written is true..in dsp2 complex only one d orbital is needed which can be done by pairing electron in Ni2+...whereas in octahedral complexes two d orbitals are used which cannot be done even by pairing of electrons in Ni2+.... so it has to form outer spin complex..config of Ni( 4s2 3d8) and config of Ni2+ (4s°3d8) so on pairing also only one d orbital can remain empty....
Ans 2 ..it shud be an inner outer complex...
Ans 3 ..plz give an example..are u talking coz none of the element has d4 configuration in ground state in the 3d series....
Ans 4..oxidation state of Cr is 0 in this complex..coordination number 12 (says J D Lee)..dunno abt the EAN...i think it shud be 36....not sure abt it...
Ans..5 the order shud be [V(CO)6]- < [Cr(CO)6] < [Mn(CO)6]
Ans 6 A..all other complexes are satisfying the EAN rule except a..in a the EAN is 35...so it will get reduced to satisfy the EAN rule...
Ans 8 A...
Ans 9 Platinum shud be in +4 oxidation state..name : Xenon hexafluorido plantinate(IV)..
Ans 10...+2..it is formed by the reaction of sodium nitroprusside with sodium sulphide...
3. I know none of the complexes have d4 (ground state)..
I have no specific example..
anyways take this..
Cr (2+)
Will there be any pairing?
1. thx govind
2. even our sir said that it is outer orbital bcz........ (he doesnt know)
4. thx everyone
5. pls explain reason govind
6. thx govind
7.
8. ans given (d)
9. ans given: xenonhexafluoroplatinate(V)
10. thx
quote: from http://www.chemicalforums.com/index.php?topic=39533.msg151651#msg151651
Well, I can only add that the [Co(H2O)6]3+ ion is somewhat of a weirdo. It is the only first row transition metal that forms a low spin complex with the weak field H2O ligand (the other first row transition metals need a strong field ligand to form a low spin complex). Other than that, the book I used discouraged the use of hybrid orbitals when working with MO diagrams, so I am no help there
Additional Info..
Cu2+ forms dsp2 square planar complexes with CN- and NH3
And abt the abv info given by asish..actually there is a line in NCERT saying that [Co(C2O4)3]3- is an inner orbital complex and is dimagnetic..though C2O42- is a weak ligand compared to H2O...by general rule it has to be outer....another exception..it's chemistry..
Reason for the abv exception given by J D Lee... high crystal field stability of the configuration t2g6 eg0 ..... only [CoF6]3- (exception to the abv exception : D) is an outer orbital paramagnetic compound
Ans 7 D,G,H...
A,B,C are false coz coordination number is determined by sigma bonds..
D is true..as sed abv..
E metal exhibit variable coordination numbers..
F it is not equal to the ligands binded to the metal..as there are two types of valencies..primary and secondary and only secondary determines the coordination number
G ..it refers to the EAN rule..
H...looks true..