Mujhe .."which indicator to use" me bahut din se doubt hai... :(
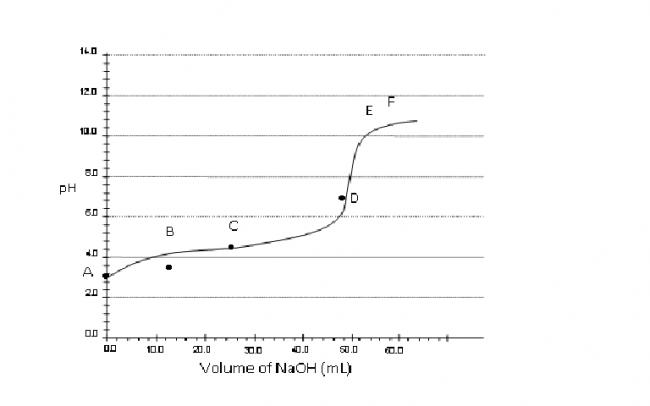
Consider a pH titration of 50 mL 0.1 M benzoic acid vs 0.1M NaOH at 25°C. The experimentally observed curve is shown below, on which points A, B, C…… F are marked.
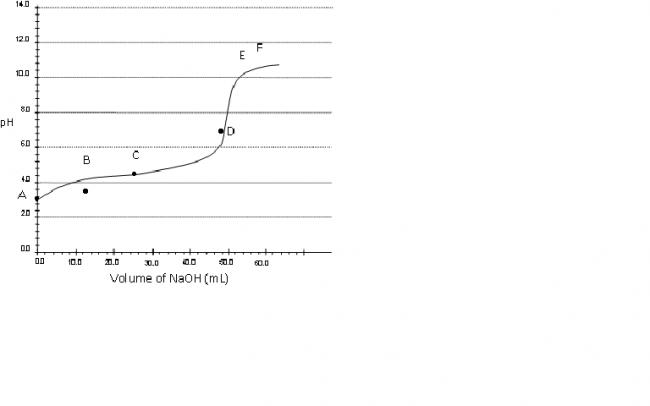
a) Which point corresponds to approximately 0.07 M
benzoic acid solution?
b) Suppose you are carrying out a conventional titration of benzoic acid and NaOH. Using choose an appropriate indicator from the table below.the answer obtained
Indicator pH range Colour change
Methyl yellow 2.9 - 4.0 red-yellow
Methyl orange 3.1 – 4.4 red-orange
Phenolphthalein 8.0 - 9.8 colorless-red
-
UP 0 DOWN 0 0 39

39 Answers
To find the equivalence point Phenolphthalein should be used as seen from the curve
We have to use such indicator... (whose range includes..neutral point ph=7).. am i right..??
@ priyam....a little mistake...be careful with this
let the volume added be V
milliequivalent of acid =5
milliequivalent of NaOH =0.1*V=V/10
remaining meq of acid=5-V/10
total volume=V+50
(5-V/10)/(V+50)=0.07=7/100
500V-10V=7V+350
17V=150
V=9 ml
Q.1 Point B.
Benzoic acid is taken in flask and then NaOH is added..
Now 0.07 M benzoic acid means... 0.03(50) M.eq is used up by NaOH..
M.eq of NaOH=0.03(50)
0.1(v)=1.5
V=15ml NaOH
so point B
@manish bhaiya
hmm.... lekin issse koi point nahi aa raha q me....
For second one
at volume of 50 ml pH=7
if you add 1 drop more the pH becomes 9 etc.
so you will be able to see the change in colour of indicator
tapanmast
#3 Posted 10:41pm 16-03-09
Re: definitely a QOD...
USE PHENOLPHTHALEIN
Phenolphthalein 8.0 - 9.8 colorless-red
means
Phenolp is colorless for pH<8
and red for pH>9.8
part b seems incomplete because it is not asking for the equivalence point
I think there is something missing from the question
please find it
I saw the Incho paper
they are asking for the half equivalence point
now try guys
yes MATRIX it is
i noe ppl lyk u never go wrong
but bro post d soln 2 and hv a luk at second ques
yes it is .....
explaination de de mere bhai
dis ques wasnt meant for ur practic but was my doubt