1st one is a jee Qn i suppose
1)A graph is plotted between PVm on y-axis and P on x-axis. The intercept is (Vm is molar volume).
a)R
b)R+a/b
c)RT
d)RT-b/a
2)
a)A ring shaped tube contains two ideal gases with molecular weights 32 and 28. Both these gases are separated by one fixed partition and another movable stopper ‘s’ which can move freely without friction inside the ring.
If both the gases have same mass,
then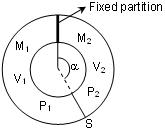
P1 + P2 = 1atm
P1 = 28/32P2
P1 = 32/28p2
P1 = P2
b) The angle ‘α’ shown in figure is (assume masses of both gases to be equal)
168°
132°
146°
180°
plz explain how u reached to the answer ..
-
UP 0 DOWN 0 0 4

4 Answers
1. C
(considering an2/ Vm to be negligible...otherwise the eqn will become complicated.
2. The movable stopper 's' will be at equillibrium, when pressure on both ends is same.
so P1=P2
and as mass are same using ideal gas eqn
PVM=constant
When takin volume A* α/2Π*R where α is variable
so
PαM = constant