is answer to Q2 -(b)
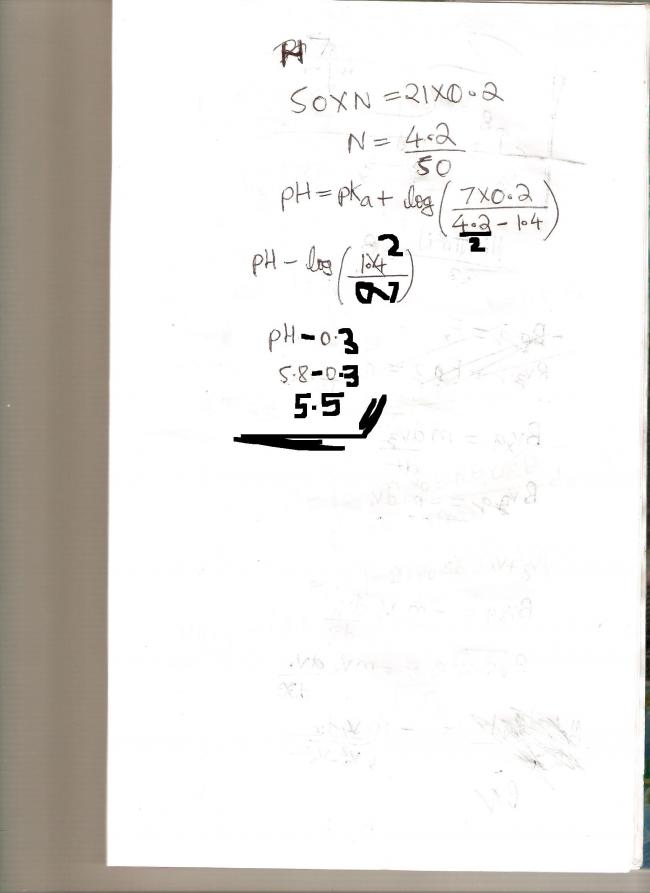
Ques1) 50 ml of a weak monobasic acid (HA) requires 21 ml 0.2 M NaOH for complete equivalence. If pH of sol n upon addition of 7 mL of this alkali to 25 mL of the above soln of HA is 5.8 , then the pKa of the acid is
(a) 6.1 (b) 5.8 (c) 5.5 (d) 6.4
Ques2) To a 200 ml of 0.1 M weak acid HA soln 90 ml of 0.1 M soln of NaOH be added. Now what volume of 0.1 M NaOH be added into the above soln so that pH of resulting soln be 5 [dissociation const Ka of (HA) is 10 ^ -5 ]
(a)2 ml (b) 20 ml (c) 10 ml (d) 15 ml.
-
UP 0 DOWN 0 0 10

10 Answers
sorry a small calculation mistake......ans is (C)
HA + NaOH → NaA + H2O
initial milimoles 20 9 +V/10 0
final 20-(9+V/10) 0 9+V/10
now since ot is an acidic buffer
pH =PKa +log[salt]/[acid]
5=5 +log[salt]/[acid]
[salt]=[acid]
[20-(9+V/10) ]/290+V = [9+V/10]/290+V
v=10ml
i took log 2 as 0.6 by mistake and i didnt see that 25mL thats y i got 6.4 before!!!!! sorry..