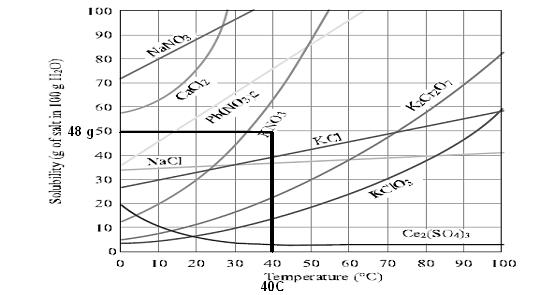
the solution is unsaturated .
since at 40°C The solution can have ≈60 g KCl dissolved.
Use data from Figure to determine whether a solution containing 48 g of KNO3 per 100 g of water at 40 °C is saturated, unsaturated, or supersaturated
-
UP 0 DOWN 0 0 2

2 Answers
Anirudh Kumar
·2009-12-10 02:32:31
lalit
·2009-12-11 01:36:23
48 gm of kno3 is unsaturated because on increasing further temperature the solubility graph attain value more than 48gm per 100 ml
which clearly shows that more kno3 can be dissolved in solution so it is unsaturated solution