ya its crrct
this is the basic defination of solute -its that component of binary homogenous mixture which is present in less amt
Assumin that all the users hav 2008 paper on their PC s .........
IN the Comprehension on "SOLUTIONS" HOW DO DETERMINE WICH IS TO BE TAKEN AS SOLUTE annd wich as SOLVENT ehtanol or water ???
Does that depend on quantity of each present??
-
UP 0 DOWN 0 0 26

26 Answers
@ tapan
amount is based on volume.....
becoz in soln phase we should concentrate on volume
Y mol fractn.......
198g of H2 mein agar 1 ek graam Uranium dala to Ur ACTS AS A SOLVENT [11] aisa kya ??
r u sure ??
if they say 2.5% (mass/volume) wats hud we infer frm this
inference = 2.5 /100 (mass/vol)
=> in 100ml solution, there is 2.5 gm of that stuff.
similarly if given mass/mass .. then we wud have said: 2.5 gm in 100 gm solution.
m always confused in dat topic .i eman percnt by mass n percnt by volume
if they say 2.5% (mass/volume) wats hud we infer frm this
(tapan m sorry bt it was replated so posted ma query here ..........sorry )
One more clarification pl...............
DEFINITION of SOLVENT says :
amount of A > amt of B -------------> basic defn of solvent.
AMT. in refernce to MASS OR VOLUME ???
actually if a solution consists of two components A and B ...
then,
we call A as the solvent .. only n only when
amount of A > amt of B -------------> basic defn of solvent.
and the whole question was based on only this defn... :)
pl. read the solution toooo.............
is it incrrct or wat??
the quantity in excess should be taken as solvent
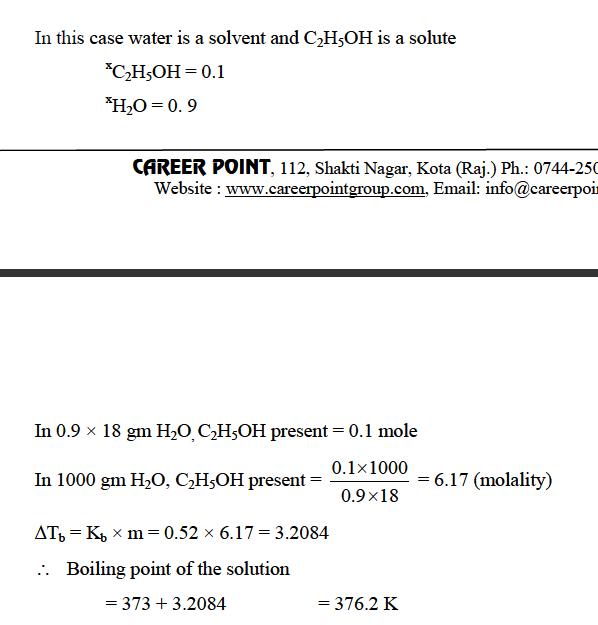
hey wat i thought was first to mole fraction as most of the people has defined above tht s less conc wala solute hai n one more thing the solute is non disosiative n water is 100% non dissosiative so its the solute
actually i did dis q bt abhi maine q gum kar diya so how can i help without q [2]
Sir, ethanol bhi to dilute hota hai .........
Jo pine layak hota hai woh bhi to dil. karte hain....... [3]
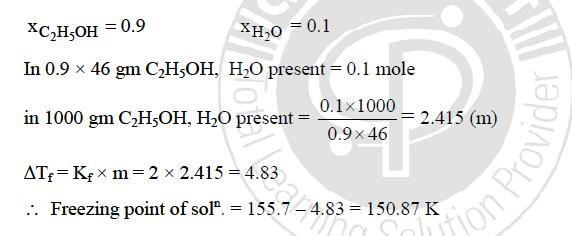
yes here water is solute and ethanol is solvent
This can be known from the question itself----In the question, it is mentioned dilute solution.......so how can solution be dilute in ethanol is the solute
the quantity in excess should be taken as solvent ( according to brilliant - tutorials )))))))
according to basics i have learnt in solution chapter,we must take ethanol as solvent.
I took water as the solvent and got wild answers
so i guess we must take etOH as the solvent!!