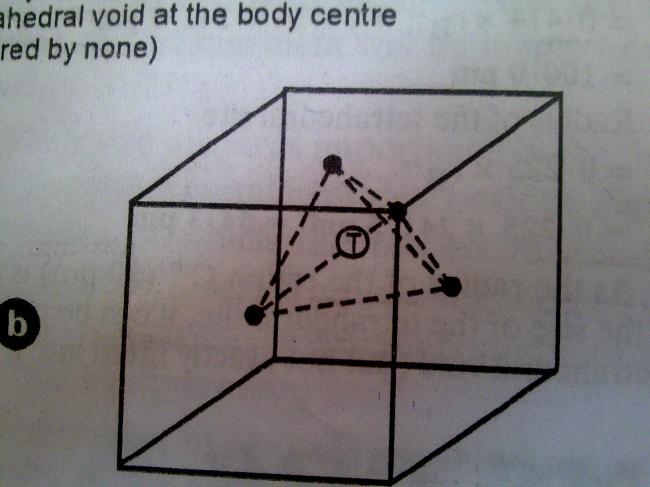
yaar dekh a cubic void will be generated
a=2R
r+R=a√3/2
r+R=R√3
r=R(√3-1)
r=R(√3-1)
r=0.732R
Consider a unit cell in which atoms will occupy all corner points, all face centres, body centre and also all edge centres of the cube.
Q65) The radius ratio of the voids (rvoid/ratom) is
a) 0.366
b) 0.414
c) 0.225
d) 0.732
ans : d
source : PT1 ;)
-
UP 0 DOWN 0 0 12

12 Answers
HELP guyzzzz!!!
its actually quite simple, but I m not able to picturise which all atoms will b in direct contact ??? [7][7]
ek silly questn.......
how do u know ke yeh void kaha par banega???
ther will b a void bw ne two adjacent face centred atoms also na....
then how to know which void so gonna counted????????
tapan I dont think you are visualising correctly.. or may be i am not understaind your question properly.!
I was talkin of the tetrahedral void created by three face centre atoms
the calculation as done by manipalmp(if I'm not wrong) refers to a void b/w body centre atom and the corner atoom
that calculation i understood.......
but the doubt remains wer 2 visualise/locate the "VOID" [2]
yaar sachi baataon to isko visualize karna mushkil hai
but put all ur conc coz isko paper pe 2d banane mein koi faida nahi hai
now i could recommend u to read thoroughly pg7-20 of Ncert
try to relate it with ur ques
if that doesn't help dont worry we r here only
whatever study material u have
please go through it once to get conceptual clarity
this was easy if you visualize it correctly
this is nothing but combination of 8 simple cubic unit cell
so consider one simple cubic unit cell and you will get the answer