quite obviously, ans 1 looks to be (d)...isn't it ?
Q1 which is not covalent
a)CO2
b)SiO2
c)BaO
d)CsNO3
Q2 In SiO2 what is coordination no. of Si and O ??
Q3 **deleted **
Q4 The [1 1 1] plane is parallel to which plane in 3D space ?
Q5 Find miller indices of plane with intercepts 0.5a,0.25b,1.5c ??
*whats this miller indice thing ??
Q6 A binary solid has Zn blend strcture with B- ions constituiting lattice and A+ occupying 25% tetrahedral voids.Whats the formula ??
Q7 A FCC corner atom in HCP is shared by how many unite cells ?
Q8what fraction of voids are occupied by carbon atom in diamond having FCC structure and sp3 hybridised ?
*whats significance of sp3 here ?
-
UP 0 DOWN 0 1 24

24 Answers
Ans 6) When particles are close-packed resulting in either ccp or hcp structure, two types of voids are generated. While the no. of octahedral voids present in a lattice is equals to the no. of colse packed structure, the no. of tetrahedral voids generated is twice this number.
Now, the total no. of tetrahedral voids formed is equals to twice the no. of atoms of element B and only 25 % = (1/4) of these are occupied by the atoms of element A. Hence the ratio of no. of atoms of A and B is 2 X (1/4) = (1/2) and thus the formula of the compound is A B 2.
Ans 8) 74 % ??????
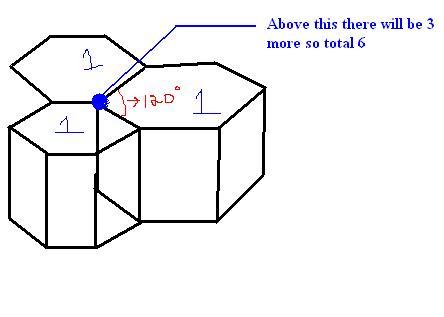
HCP arrangement ... is the one i have drawn.. This is also FCC (face centred cubic) corner atom is the blue one
thx asish....
@debo..
arre yaar main kya karron..main khud confused hoon in answers mein...
ans1=a,c,d
Q4. [1 1 1] means the reciprocal of the intercepts simplified to the lowest ration is (1,1,1) .i.e. the intercepts are (1,1,1)..
So the plane parallel to this plane is x+y+z=c where c is a const..
what is that ?
can any of CO2 or SiO2 be ionic ????
....you say its not (c) nor (d)...then its going to be (m) or (n) or.....[4]
@debo
No debo...ans is diff
@asish
i suppose u r answering Q4...
i dont have answer for that ques...can u xplain how ?
@uttara
ans 7 rite...plz explain
and edited Q6,deleted Q3...
@tush,ans 8 is wrong
@saket,ans 1 is wrong
Ans 5) coeff of a,b, and c are 0.5, 0.25 and 1.5
Reciprocal of coeff of a,b and c are 2 , 4 , 2/3
Therefore, multiplying the reciprocal of coefficients of a,b, and c by 3 , we have (6 , 12 , 2)
Hence Miller Indices are (3,6,1) and this face is represented as (3 6 1). [Taking 2 common]