A container fitted with frictional massless piston consist of five values – I, II, III, IV and V. These valves open automatically if pressure exceed 1.5,2.2,2.5,4.4 and 4.8 atm respectively. Under the given initial conditions (mentioned in given diagram ) system is in state of equilibrium. Piston is now pressed in downward direction very slowly.
[Note: Consider the diameter of value tube negligible and temperature remain constant]
(i) Value – II will be opened first
(ii) As the piston crosses the value which will be opened first, the remaining number of moles in container are 5/3
(iii) Value – V will be the second valve which will open
(iv) Number of moles will zero as piston crosses Valve – V
How many of the below given statements are correct: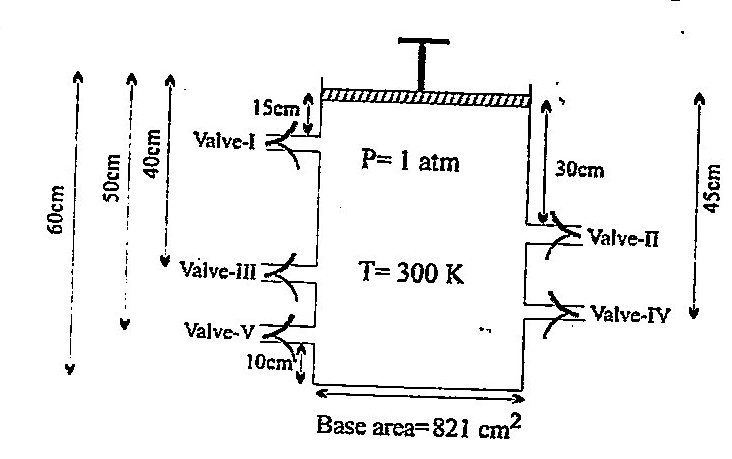
-
UP 0 DOWN 0 0 0
