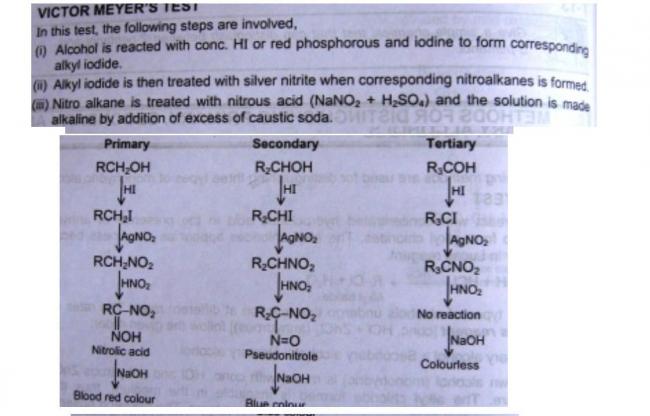
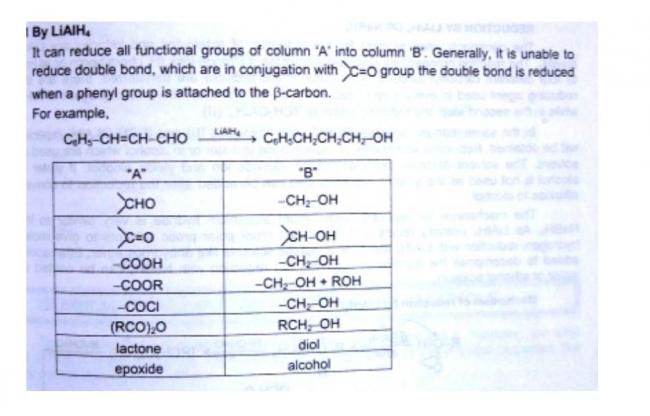
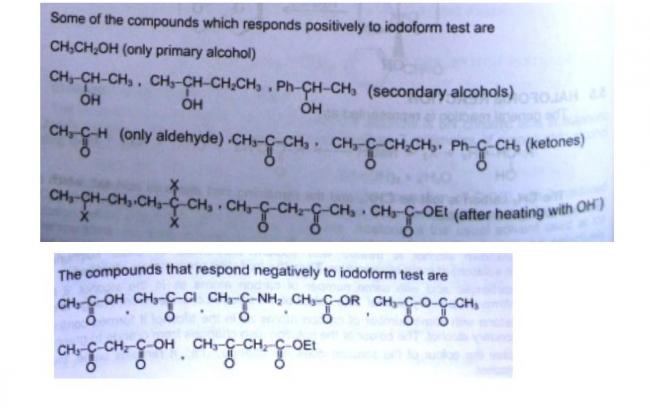
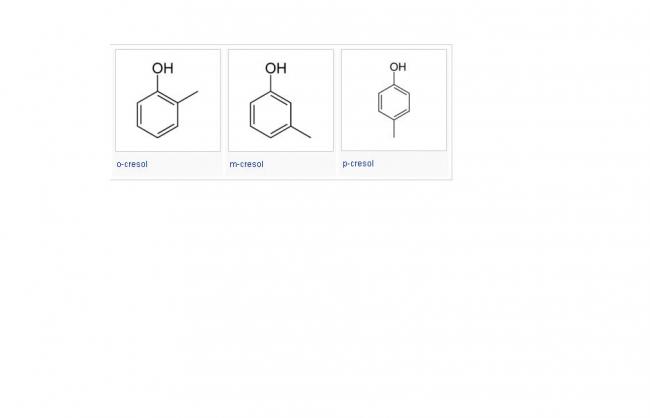
Stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during the non-stereospecific creation of a new stereocenter or during the non-stereospecific transformation of a pre-existing one. The selectivity arises from differences in steric effects and electronic effects in the mechanistic pathways leading to the different products. Stereoselectivity can vary in degree but it can never be total since the activation energy difference between the two pathways is finite. However, in favorable cases, the minor stereoisomer may not be detectable by the analytic methods used.
An enantioselective reaction is one in which one enantiomer is formed in preference to the other, in a reaction that creates an optically active product from an achiral starting material, using either a chiral catalyst, an enzyme or a chiral reagent. The degree of selectivity is measured by the enantiomeric excess. An important variant is kinetic resolution, in which a pre-existing chiral center undergoes reaction with a chiral catalyst, an enzyme or a chiral reagent such that one enantiomer reacts faster than the other and leaves behind the less reactive enantiomer, or in which a pre-existing chiral center influences the reactivity of a reaction center elsewhere in the same molecule.
A diastereoselective reaction is one in which one diastereomer is formed in preference to another (or in which a subset of all possible diastereomers dominates the product mixture), establishing a prefered relative stereochemistry. In this case, either two or more chiral centers are formed at once such that one relative stereochemistry is favored, or a pre-existing chiral center (which needs not be optically pure) biases the stereochemical outcome during the creation of another. The degree of relative selectivity is measured by the diastereomeric excess.
Stereoconvergence can be considered an opposite of stereoselectivity, when the reaction of two different stereoisomers yield a single product stereoisomer.
Stereospecific reaction
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one (or a subset) of the stereoisomers.
In contrast, stereoselectivity is the property of a reactant mixture where a non-stereospecific mechanism allows for the formation of multiple products, but where one (or a subset) of the products is favored by factors, such as steric access, that are independent of the mechanism.
A stereospecific mechanism specifies the stereochemical outcome of a given reactant, whereas a stereoselective reaction selects products from those made available by the same, non-specific mechanism acting on a given reactant. Given a single, stereoisomerically pure starting material, a stereospecific mechanism will give 100% of a particular stereoisomer (or no reaction), although loss of stereochemical integrity can easily occur through competing mechanisms with different stereochemical outcomes. A stereoselective process will normally give multiple products even if only one mechanism is operating on an isomerically pure starting material.
The term stereospecific reaction is ambiguous, since the term reaction itself can mean a single-mechanism transformation (such as the Diels-Alder reaction), which could be stereospecific, or the outcome of a reactant mixture that may proceed through multiple competing mechanisms, specific and non-specific. In the latter sense, the term stereospecific reaction is commonly misused to mean 'highly stereoselective reaction'.
Chiral synthesis is built on a combination of stereospecific transformations (for the interconversion of existing stereocenters) and stereoselective ones (for the creation of new stereocenters), where also the optical activity of a chemical compound is preserved.
Examples
• Nucleophilic substitution at sp3 centres can proceed by the stereospecific SN2 mechanism, causing only inversion, or by the non-specific SN1 mechanism, the outcome of which can show a modest selectivity for inversion, depending on the reactants and the reaction conditions to which the mechanism does not refer. The choice of mechanism adopted by a particular reactant combination depends on other factors (steric access to the reaction centre in the substrate, nucleophile, solvent, temperature). For example, tertiary centres react almost exclusively by the SN1 mechanism whereas primary centres (except neopentyl centres) react almost exclusively by the SN2 mechanism. When a nucleophilic substitution results in incomplete inversion, it is because of a competition between the two mechanisms, as often occurs at secondary centres, or because of double inversion (as when iodide is the nucleophile).
• The addition of carbenes to alkenes is stereospecific in that the geometry of the alkene is preserved in the product. For example, dibromocarbene and cis-2-butene yield cis-2,3-dimethyl-1,1-dibromocyclopropane, whereas the trans isomer exclusively yields the trans cyclopropane.
This addition remains stereospecific even if the starting alkene is not isomerically pure, as the products' stereochemistry will match the reactants'.
• The disrotatory ring closing reaction of conjugated trienes is stereospecific in that isomeric reactants will give isomeric products. For example, trans,cis,trans-2,4,6-octatriene gives cis-dimethylcyclohexadiene, whereas the trans,cis,cis reactant isomer gives the trans product and the trans,trans,trans reactant isomer does not react in this manner.
Regioselective reaction
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions . It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where on a substituted benzene ring a further substituent will add.
A specific example is a halohydrin formation reaction with 2-propenylbenzene :
The reaction product is a mixture of two isomers and the regioselectivity is said to be poor.
Regioselectivity in ring-closure reactions is subject to Baldwin's rules.
A synthon is a concept in retrosynthetic analysis. It is defined as a structural unit within a molecule which is related to a possible synthetic operation. The term was coined by E.J. Corey. It is noted that the phrase does not feature very prominently in Corey's book, The Logic of Chemical Synthesis, as it is not included in the index.
In planning the synthesis of phenylacetic acid, two synthons are identified: a nucleophilic "-COOH" group, and an electrophilic "PhCH2+" group. Of course, both synthons do not exist per se; synthetic equivalents corresponding to the synthons are reacted to produce the desired product. In this case, the cyanide anion is the synthetic equivalent for the -COOH synthon, while benzyl bromide is the synthetic equivalent for the benzyl synthon.
The synthesis of phenylacetic acid determined by retrosynthetic analysis is thus:
1. PhCH2Br + NaCN → PhCH2CN + NaBr
2. PhCH2CN + 2 H2O → PhCH2COOH + NH3