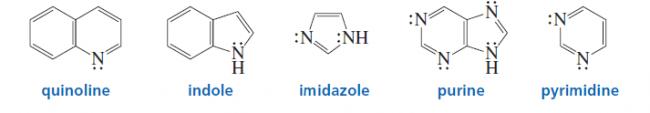
9) about E1
An E1 reaction is regioselective. The major product is
the most stable alkene, which is generally the most substituted
alkene. An E1 reaction is stereoselective. The major
product is the alkene with the bulkiest groups on opposite
sides of the double bond. The carbocation formed in the
first step can undergo both syn and anti elimination; therefore,
the two groups to be eliminated in a cyclic compound
do not have to be trans or both in axial positions. Alkyl substitution
increases the stability of a carbocation and decreases
the stability of a carbanion.
AN ATTEMPT TO HELP
1) Stereochemistry of diel's alder reaction :
If a Diels–Alder reaction creates an aymmetric carbon in the product, identical
amounts of the R and S enantiomers will be formed. In other words, the product will be
a racemic mixture .
The Diels–Alder reaction is a syn addition reaction with respect to both the diene
and the dienophile: One face of the diene adds to one face of the dienophile.
Therefore if the substituents in the dienophile are cis, they will be cis in the product;
if the substituents in the dienophile are trans, they will be trans in the product.
The substituents in the diene will also maintain their relative configurations in the
products. Notice that compounds containing carbon–carbon triple bonds can also be
used as dienophiles in Diels–Alder reactions to prepare compounds with two isolated
double bonds.
Because only syn addition occurs, each reaction forms only two of the stereoisomers.
The Diels–Alder reaction is stereospecific—the configuration of the reactants is
maintained during the course of the reaction—because the reaction is concerted.
If both the diene and the dienophile are unsymmetrically substituted two products are possible.
Which of the two products will be formed (or will be formed in greater yield) depends on the charge distribution in each of the reactants.
To determine the charge distribution, we need to draw contributing resonance structures.
-
UP 0 DOWN 0 8 50

50 Answers
Niice manmay! Keep up the good work! :)
Also, in 7), if the size of the nucleophile is large enough, it can tear through the envelope of protic solvent molecules and perform its function. That is why for halogens the order of nucleophilicity in polar protic solvents is
F < Cl < Br < I as per size and electronegativity.
8) about E2
An E2 reaction is regioselective. The major product is the
more stable alkene, unless the reactants are sterically hindered
or the leaving group is poor. The more stable alkene is generally (but not always) the more substituted alkene. The more substituted alkene is predicted by Zaitsev’s rule: It is
the alkene formed when a proton is removed from the
\beta -carbon that is bonded to the fewest hydrogens.
An E2 reaction is stereoselective: If the \beta-carbon has two
hydrogens, both E and Z products will be formed, but the
one with the bulkiest groups on opposite sides of the double
bond is more stable and will be formed in greater yield. Anti
elimination is favored in an E2 reaction. If the -carbon has
only one hydrogen, only one alkene is formed, since there is
only one conformer in which the groups to be eliminated are
anti. If the reactant is a cyclic compound, the two groups to
be eliminated must be trans to one another; in the case of
six-membered rings, both groups must be in axial positions.
Elimination is more rapid when H and X are diaxial in the
more stable conformer.
10) Reactivity of alcohols and ether
Alcohols and ethers have leaving groups(OH-, RO-) that are stronger bases than
halide ions(X-). Alcohols and ethers, therefore, are less reactive than alkyl halides in
substitution and elimination reactions. We will see that because their leaving groups
are strongly basic, alcohols and ethers have to be “activated†before they can undergo
a substitution or an elimination reaction. In contrast, sulfonates and sulfonium salts
have weakly basic leaving groups so they undergo substitution reactions with ease.
Because the OH group of the alcohol has to be protonated before it can be displaced by
a nucleophile, only weakly basic nucleophiles( I-, Br-, Cl-) can be used in the substitution
reaction. Moderately and strongly basic nucleophiles (NH3 , RNH2 , CH3O-)
would be protonated in the acidic solution and, once protonated, would no longer be
nucleophiles( NH4+, RNH3+ ) or would be poor nucleophiles( CH3OH ).
Primary, secondary, and tertiary alcohols all undergo nucleophilic substitution
reactions with HI, HBr, and HCl to form alkyl halides.
11) Lucas test
Whether an alcohol is primary, secondary, or tertiary
can be determined by taking advantage of
the relative rates at which the three classes of alcohols react
with HCl/ZnCl2. This is known as the Lucas test. The alcohol
is added to a mixture of HCl and ZnCl2—the Lucas reagent.
Low-molecular-weight alcohols are soluble in the Lucas
reagent, but the alkyl halide products are not, so the solution
turns cloudy as the alkyl halide is formed. When the test is carried
out at room temperature, the solution turns cloudy immediately
if the alcohol is tertiary, turns cloudy in about five minutes
if the alcohol is secondary, and remains clear if the alcohol is
primary. Because the test relies on the complete solubility of
the alcohol in the Lucas reagent, it is limited to alcohols with
fewer than six carbons.
12) Dehydration of alcohol
An acid always reacts with an organic molecule in the same way: It protonates the
most basic (electron-rich) atom in the molecule. Thus, in the first step of the dehydration
reaction, the acid protonates the oxygen atom of the alcohol. As we saw earlier,
protonation converts the very poor leaving group( OH- ) into a good leaving group( H2O )
In the next step, water departs, leaving behind a carbocation. A base in the
reaction mixture (water is the base in highest concentration) removes a proton from a
\beta-carbon (a carbon adjacent to the positively charged carbon), forming an alkene and
regenerating the acid catalyst. Notice that the dehydration reaction is an E1 reaction of
a protonated alcohol.
When more than one elimination product can be formed, the major product is the
more substituted alkene—the one obtained by removing a proton from the -carbon
that is bonded to the fewest hydrogens. The more substituted alkene is the major product because it is the more stable alkene, so it has the more stable transition state leading to its formation.
2) conformations of the diene
A conjugated diene can exist in two different planar
conformations: an s-cis conformation and an s-trans conformation. By “s-cis,â€
we mean that the double bonds are cis about the single bond(s = single). The
s-trans conformation is little more stable (2.3 kcal or 9.6 kJ) than the s-cis conformation
because the close proximity of the hydrogens causes some steric strain
.The rotational barrier between the s-cis and s-trans conformations
(4.9 kcal/mol 20.5 kJ/mole) is low enough to allow them to interconvert rapidly
at room temperature.
In order to participate in a Diels–Alder reaction, the conjugated diene must be in an
s-cis conformation because in an s-trans conformation, the number 1 and number 4
carbons are too far apart to react with the dienophile. A conjugated diene that is locked
in an s-trans conformation cannot undergo a Diels–Alder reaction.
A conjugated diene that is locked in an s-cis conformation, such as 1,3-cyclopentadiene,
is highly reactive in a Diels–Alder reaction. When the diene is a cyclic compound,
the product of a Diels–Alder reaction is a bridged bicyclic compound—a compound
that contains two rings that share two nonadjacent carbons.
14) reaction of epoxides
Although an epoxide and an ether have the same leaving group, epoxides are much
more reactive than ethers in nucleophilic substitution reactions because the strain in
the three-membered ring is relieved when the ring opens . Epoxides,
therefore, readily undergo ring-opening reactions with a wide variety of nucleophiles.
Epoxides, like other ethers, react with hydrogen halides. In the first step of the reaction,
the nucleophilic oxygen is protonated by the acid. The protonated epoxide is
then attacked by the halide ion. Because epoxides are so much more reactive than
ethers, the reaction takes place readily at room temperature. (Recall that the reaction
of an ether with a hydrogen halide requires heat.)
Protonated epoxides are so reactive that they can be opened by poor nucleophiles, such
as H2O and alcohols. ( HB+ is any acid in the reaction mixture; B: is any base.)
If different substituents are attached to the two carbons of the protonated epoxide
(and the nucleophile is something other than H2O ), the product obtained from
nucleophilic attack on the 2-position of the oxirane ring will be different from that
obtained from nucleophilic attack on the 3-position. The major product is the one
resulting from nucleophilic attack on the more substituted carbon.
The more substituted carbon is more likely to be attacked because, after the epoxide
is protonated, it is so reactive that one of the C - O bonds begins to break before the
nucleophile has a chance to attack. As the C - O bond starts to break, a partial positive
charge develops on the carbon that is losing its share of the oxygen’s electrons. The
protonated epoxide breaks preferentially in the direction that puts the partial positive
charge on the more substituted carbon, because a more substituted carbocation is more
stable.
The best way to describe the reaction is to say that it occurs by a pathway that is
partially SN1 and partially SN2 It is not a pure reaction because a carbocation
intermediate is not fully formed; it is not a SN2 pure reaction because the leaving
group begins to depart before the compound is attacked by the nucleophile.
another thng i forget to mention More substituted site is attacked under acidic conditions and less substituted site is attacked under basic medium.
pritish u can assume any reaction of ur own and explain.....
i don't remmeber the reaction actually......
15) THE TOXICITY OF BENZENE
Although benzene has been widely used in chemical
synthesis and has been frequently used as a
solvent, it is toxic. Its major toxic effect is on the central nervous
system and on bone marrow. Chronic exposure to benzene
causes leukemia and aplastic anemia. A higher-than-average
incidence of leukemia has been found in industrial workers
with long-term exposure to as little as 1 ppm benzene in the atmosphere.
Toluene has replaced benzene as a solvent because,
although it is a central nervous system depressant like benzene,
it does not cause leukemia or aplastic anemia. “Glue sniffersâ€
seek the narcotic central nervous system effects of solvents
such as toluene. This can be a highly dangerous activity.
16)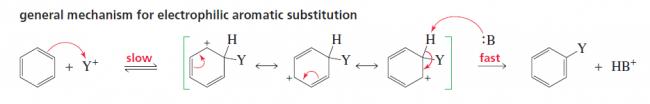
The first step is relatively slow and endergonic because an aromatic compound is
being converted into a much less stable nonaromatic intermediate . The
second step is fast and strongly exergonic because this step restores the stabilityenhancing
aromaticity.
nice thread manmay...[1][1][1][1][1][1][1]
B.T.W what is the source ???