6)
The very first conversion that I'm attempting..........so pls forgive me if I'm wrong[2]
I KEPt THE NAME OF THE QUESTION AS SUCH BECAUSE I WILL KEEP ON POSTING MY QUESTIONS IN THIS TAG ONLYYYYYYYY I WILL ADD NEW QUESTIONS DAILYYYYYYYYYYYY whats more than organic in chemistry.. so i will start with that
1]Convert aniline to phenyl acetic acid in NOT MORE THAN 5 steps....
2)Convert cyclopent2ene1one to cyclobutanol in NOT MORE THAN 5 steps....
3]give the steps for the mechanism for the conversion of cyclohexanone oxime in the presence of H+, heat to caprolactam
4]convert benzaldehyde to 3-phenylpropan-1-ol
5]what is fugisity
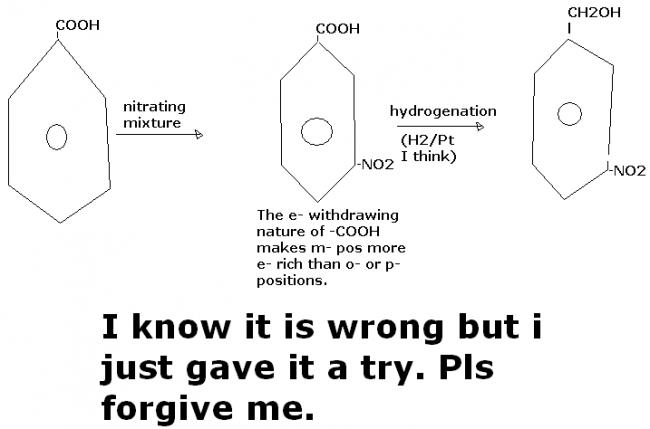
6]how will u covert benzoic acid to m-nitrobenzylalcohol?
7]what happens when tripalmitin is heated with super heated steam under pressure in the presence of dil sulphuric acid?
8]what happens when tripalmitin is boiled with KOH solution?
6)
The very first conversion that I'm attempting..........so pls forgive me if I'm wrong[2]

to convert cyclopent2-en-1one to cyclobutanol .
first react with Br2 / CCl4 , then favorski , then decarboxylation , then treat with aq.NaOH .
actually this is of 7 steps couldn't think of shorter . but it looks to be of 4 steps no ??
i mostly hope that the questions will be ready by tomorrow night.
and tell me do u lke CONCEPTUAL QUESTIONS OR CONVERSIONS?
Conversions [1]
So that every reaction has a chance to show itself
9]A hydrocarbon A (C6H10) on reduction first gives B (C6H12) anf finally C (C6H14).A on ozonolysis followed by work up with Zn-H2Ogives two molecules of aldehydes C2H4O (D) and one molcule of aldehyde E(C2H2O).oxidation of B with acidified Kmno4 gives an acid F (C4H8O2).determinestructures a to f with proper reasoning
11]DIFFERENTIATE BETWEEN EPIMERS AND ANOMERS ,,,,,
IF POSSIBLE GIVE EXAMPLES TO SUPPORT YOUR ANSWERS.
12]When 1-cyclobutyl propan-2-ol is treated with conc. (443K temp.) , predict the product
(a) 1-cyclobutyl prop-1-ene
(b) 1-methyl cyclohex-1-ene
(c) 1-ethyl cyclopent-1-ene.
please state answer stepwise & also say whether rearrangement is possible or not.
I AM STARTING PHYSICAL CHEMISTRY WITH THIS POST
14]in the absorption of carbon di oxide,caustic potash,is preffered to caustic soda WHY??????????????????
15]in an ostwald-walker experiment, dry air was first blown through a solution containing a certain amount of solute (M = 278) in 150 g of water, and then also through pure water. the loss in mass of water was found to be 0.0827g while the mass of water absorbed in sulphuric acid was 3.317g. calculate the amount of the solute
16]The dissociation pressure of solid ammonium hydrosulphide(NH4HS) at 270c is 60cm.what will be the total pressure when it dissociates at same temperature in presence of NH3 at a pressure of 45cm
18]A given sample of milk turns sour at room temperature (200C) in 64 hrs .In a refrigerator at 30C milk can be stored 3 times as long before it sours.
Estimate how long it'll take tHE milk to sour at 400C ???
19]why it is difficult to prepare pure amines by ammonolysis of alkyl halide?
20]a hydrocarbon A (C6H10) on reduction first gives B (C6H12) anf finally C (C6H14).A on ozonolysis followed by work up with Zn-H2Ogives two molecules of aldehydes C2H4O (D) and one molcule of aldehyde E(C2H2O).oxidation of B with acidified Kmno4 gives an acid F (C4H8O2).determinestructures a to f with proper reasoning
21}8. Why is pyridine aromatic? Aren't there 8 pi-electrons, violating Huckel's rule?