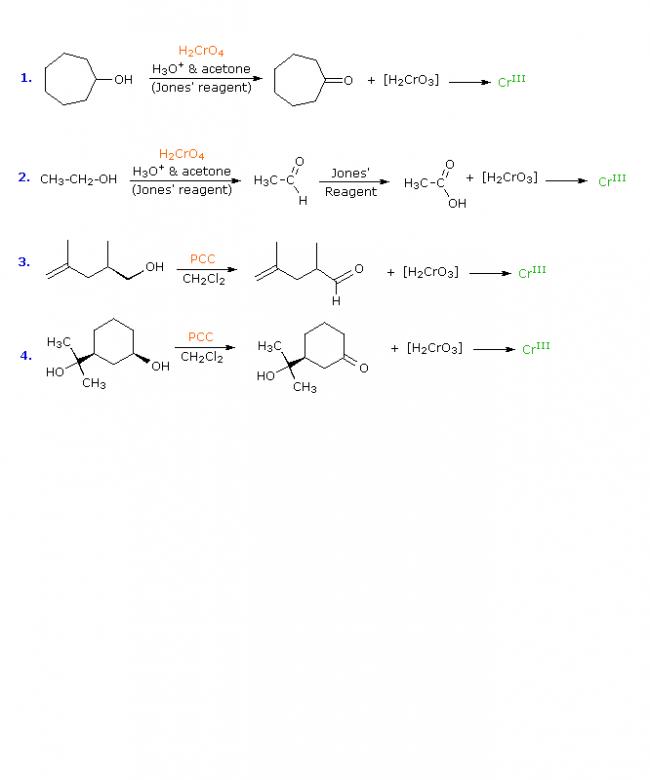
Addition reactions of isolated dienes proceed more or less as expected from the behavior of simple alkenes. Thus, if one molar equivalent of 1,5-hexadiene is treated with one equivalent of bromine a mixture of 5,6-dibromo-1-hexene, 1,2,5,6-tetrabromohexane and unreacted diene is obtained, with the dibromo compound being the major product (about 50%).
CH2=CH(CH2)2CH=CH2 + Br2 BrCH2CHBr(CH2)2CH=CH2 + BrCH2CHBr(CH2)2CHBrCH2Br + CH2=CH(CH2)2CH=CH2
5,6-dibromo-1-hexene 1,2,5,6-tetrabromohexane 1,5-hexadiene
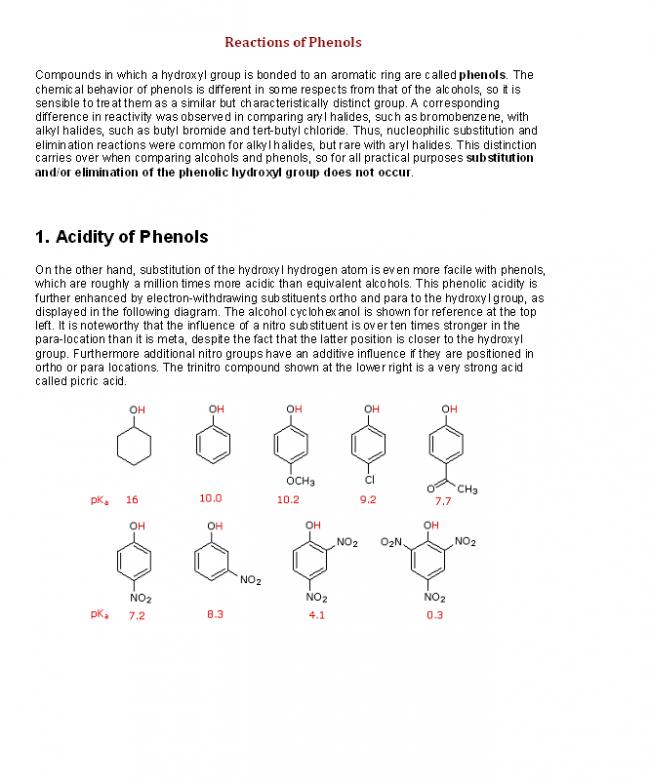
Similar reactions of conjugated dienes, on the other hand, often give unexpected products. The addition of bromine to 1,3-butadiene is an example. As shown below, a roughly 50:50 mixture of 3,4-dibromo-1-butene (the expected product) and 1,4-dibromo-2-butene (chiefly the E-isomer) is obtained. The latter compound is remarkable in that the remaining double bond is found in a location where there was no double bond in the reactant. This interesting relocation requires an explanation.
CH2=CH-CH=CH2 + Br2 BrCH2CHBr-CH=CH2 + BrCH2CH=CHCH2Br
3,4-dibromo-1-butene 1,4-dibromo-2-butene
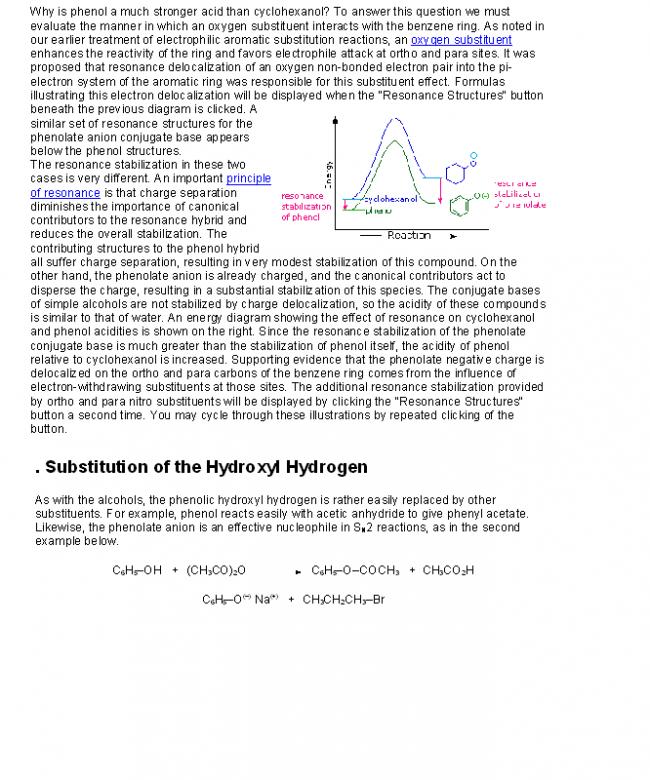
The expected addition product from reactions of this kind is the result of 1,2-addition, i.e. bonding to the adjacent carbons of a double bond. The unexpected product comes from 1,4-addition, i.e. bonding at the terminal carbon atoms of a conjugated diene with a shift of the remaining double bond to the 2,3-location. These numbers refer to the four carbons of the conjugated diene and are not IUPAC nomenclature numbers. Product compositions are often temperature dependent, as the addition of HBr to 1,3-butadiene demonstrates.
CH2=CH-CH=CH2 + HBr
reaction temperature CH3CHBr-CH=CH2 +
1,2 addition yield CH3CH=CHCH2Br
1,4 addition yield
0 ºC
40 ºC 70%
15% 30%
85%
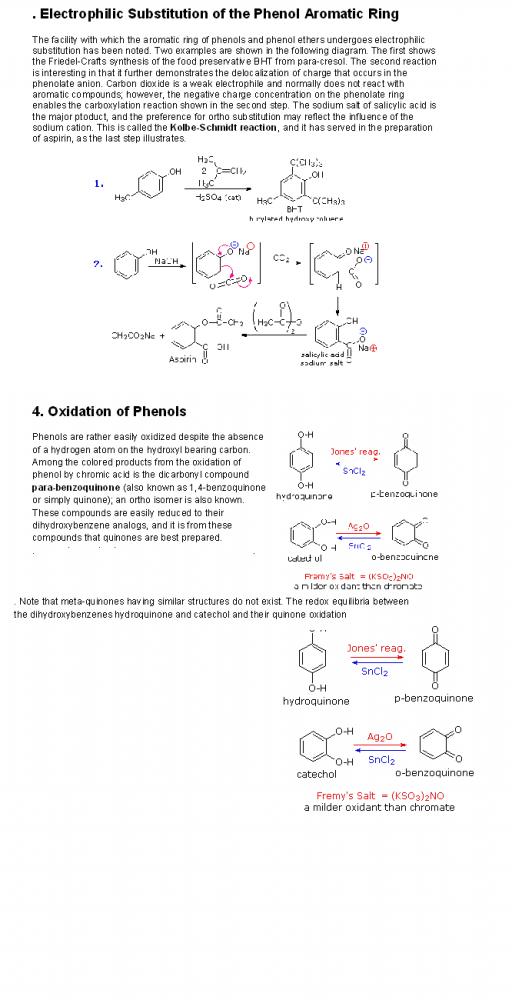
Bonding of an electrophilic atom or group to one of the end carbon atoms of a conjugated diene (#1) generates an allyl cation intermediate. Such cations are stabilized by charge delocalization, and it is this delocalization that accounts for the 1,4-addition product produced in such addition reactions. As shown in the diagram, the positive charge is distributed over carbons #2 and #4 so it is at these sites that the nucleophilic component bonds. Note that resonance stabilization of the allyl cation is greater than comparable stabilization of 1,3-butadiene, because charge is delocalized in the former, but created and separated in the latter.
. Diels-Alder Cycloaddition
The unique character of conjugated dienes manifests itself dramatically in the Diels-Alder Cycloaddition Reaction. A cycloaddition reaction is the concerted bonding together of two independent pi-electron systems to form a new ring of atoms. When this occurs, two pi-bonds are converted to two sigma-bonds, the simplest example being the hypothetical combination of two ethene molecules to give cyclobutane. This does not occur under normal conditions, but the cycloaddition of 1,3-butadiene to cyanoethene (acrylonitrile) does, and this is an example of the Diels-Alder reaction. The following diagram illustrates two cycloadditions, and introduces several terms that are useful in discussing reactions of this kind.
. Diels-Alder Cycloaddition
The unique character of conjugated dienes manifests itself dramatically in the Diels-Alder Cycloaddition Reaction. A cycloaddition reaction is the concerted bonding together of two independent pi-electron systems to form a new ring of atoms. When this occurs, two pi-bonds are converted to two sigma-bonds, the simplest example being the hypothetical combination of two ethene molecules to give cyclobutane. This does not occur under normal conditions, but the cycloaddition of 1,3-butadiene to cyanoethene (acrylonitrile) does, and this is an example of the Diels-Alder reaction. The following diagram illustrates two cycloadditions, and introduces several terms that are useful in discussing reactions of this kind.
. Diels-Alder Cycloaddition
The unique character of conjugated dienes manifests itself dramatically in the Diels-Alder Cycloaddition Reaction. A cycloaddition reaction is the concerted bonding together of two independent pi-electron systems to form a new ring of atoms. When this occurs, two pi-bonds are converted to two sigma-bonds, the simplest example being the hypothetical combination of two ethene molecules to give cyclobutane. This does not occur under normal conditions, but the cycloaddition of 1,3-butadiene to cyanoethene (acrylonitrile) does, and this is an example of the Diels-Alder reaction. The following diagram illustrates two cycloadditions, and introduces several terms that are useful in discussing reactions of this kind.
The Diels-Alder reaction is an important and widely used method for making six-membered rings, as shown on the right. The reactants used in such reactions are a conjugated diene, simply referred to as the diene, and a double or triple bond coreactant called the dienophile, because it combines with (has an affinity for) the diene. The Diels-Alder cycloaddition is classified as a [4+2] process because the diene has four pi-electrons that shift position in the reaction and the dienophile has two.
The Diels-Alder reaction is a single step process, so the diene component must adopt a cis-like conformation in order for the end carbon atoms (#1 & #4) to bond simultaneously to the dienophile. Such conformations are called s-cis, the s referring to the single bond connecting the two double bonds. The s-cis and s-trans conformers of 1,3-butadiene are shown in the preceding diagram. For many acyclic dienes the s-trans conformer is more stable than the s-cis conformer (due to steric crowding of the end groups), but the two are generally in rapid equilibrium, permitting the use of all but the most hindered dienes as reactants in Diels-Alder reactions. In its usual form, the diene component is electron rich, and the best dienophiles are electron poor due to electron withdrawing substituents such as CN, C=O & NO2. The initial bonding interaction reflects this electron imbalance, with the two new sigma-bonds being formed simultaneously, but not necessarily at equal rates.