4]Is WO4 4- a better oxidizing agent than CrO4 4- if yes or no also give me the parameters for measuring the strength of oxidizing agent!!!!
I KEPt THE NAME OF THE QUESTION AS SUCH BECAUSE I WILL KEEP ON POSTING MY QUESTIONS IN THIS TAG ONLYYYYYYYY I WILL ADD NEW QUESTIONS DAILYYYYYYYYYYYY whats more than organic in chemistry.. so i will start with that
1]Convert aniline to phenyl acetic acid in NOT MORE THAN 5 steps....
2)Convert cyclopent2ene1one to cyclobutanol in NOT MORE THAN 5 steps....
3]give the steps for the mechanism for the conversion of cyclohexanone oxime in the presence of H+, heat to caprolactam
4]convert benzaldehyde to 3-phenylpropan-1-ol
5]what is fugisity
-
UP 0 DOWN 0 9 213

213 Answers
OIL AND WATER CAN FORM A STABLE DISPERSION WITH THE HELP OF A THIRD SUBSTANCE .WHAT IS THAT SUBSTANCE?
Composition of a sample of wustite is Fe0.93 .What percentage of iron is present in the form of Fe(III)
When a bottle of perume is opened, odorous molecules mix with air and slowly diffuse throughout the entire room. which is correct for this process ?
a) G<0
b) S>0
c) H nearly equal to 0
d) S<0
1 mole of Fe0.9 O was dopped by 0.2 mole Ti4+ ions where Ti4+ replaced Fe+2 replaced Fe+2 in the crystal. this compound is dissolved in 1 litre of water & ten treated with 0.1N KMnO4 SOLUTION IN ACIDIC MEDIUM. calculate volume of KMnO4 required in ml
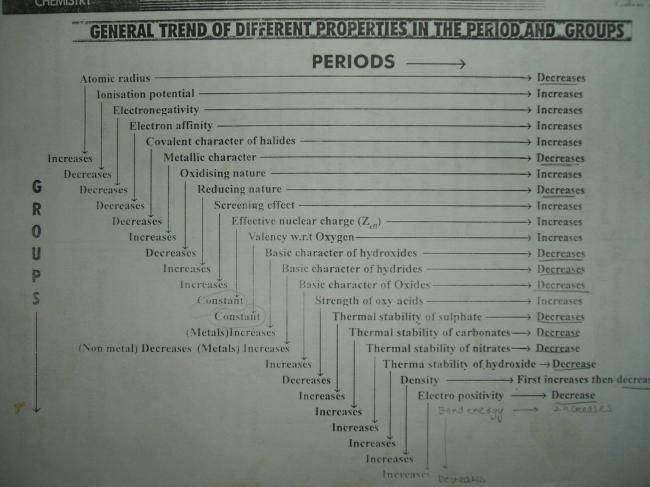
1]how larger anions are stabilised by larger cations through lattice energy effects.....is the reason dat superoxide ion o2- is stabilised by large cations like potassium,rubidium...
2]Expain the trend of boiling point & melting point BF3
3) dIAMOND HAVE BETTER THERMAL CONDUCTIVITY THAN Cu. Why?
Catalysts
With rare exception, no reaction below 480 °C occurs between H2 and organic compounds in the absence of metal catalysts. The catalyst binds both the H2 and the unsaturated substrate and facilitates their union. Platinum group metals, particularly platinum, palladium, rhodium, and ruthenium, form highly active catalysts, which operate at lower temperatures and lower pressures of H2. Non-precious metal catalysts, especially those based on nickel (such as Raney nickel and Urushibara nickel) have also been developed as economical alternatives, but they are often slower or require higher temperatures. The trade-off is activity (speed of reaction) vs. cost of the catalyst and cost of the apparatus required for use of high pressures. Notice that the Raney-nickel catalysed hydrogenations require high pressures:[4][5]
Two broad families of catalysts are known - homogeneous catalysts and heterogeneous catalysts. Homogeneous catalysts dissolve in the solvent that contains the unsaturated substrate. Heterogeneous catalysts are solids that are suspended in the same solvent with the substrate or are treated with gaseous substrate.
[edit] Homogeneous catalysts
Illustrative homogeneous catalysts include the rhodium-based compound known as Wilkinson's catalyst and the iridium-based Crabtree's catalyst. An example is the hydrogenation of carvone: [6].
Hydrogenation is sensitive to steric hindrance explaining the selectivity for reaction with the exocyclic double bond but not the internal double bond.
The activity and selectivity of homogeneous catalysts is adjusted by changing the ligands. For prochiral substrates, the selectivity of the catalyst can be adjusted such that one enantiomeric product is favored. Asymmetric hydrogenation is also possible via heterogeneous catalysis on a metal that is modified by a chiral ligand.[7]
Homogeneous catalysts are less active than heterogeneous catalysts.
[edit] Heterogeneous catalysts
Heterogeneous catalysts for hydrogenation are more common industrially. As in homogeneous catalysts, the activity is adjusted through changes in the environment around the metal, i.e. the coordination sphere. Different faces of a crystalline heterogeneous catalyst display distinct activities, for example. Similarly, heterogeneous catalysts are affected by their supports, i.e. the material upon with the heterogeneous catalyst is bound. In many cases, highly empirical modifications involve selective "poisons." Thus, a carefully chosen catalyst can be used to hydrogenate some functional groups without affecting others, such as the hydrogenation of alkenes without touching aromatic rings, or the selective hydrogenation of alkynes to alkenes using Lindlar's catalyst. For example, when the catalyst palladium is placed on barium sulfate and then treated with quinoline, the resulting catalyst reduces alkynes only as far as alkenes. The Lindlar catalyst has been applied to the conversion of phenylacetylene to styrene.[8]
Asymmetric hydrogenation is also possible via heterogeneous catalysis on a metal that is modified by a chiral ligand.[7]
[edit] Hydrogen sources
For hydrogenation, the obvious source of hydrogen is H2 gas itself, which is typically available commercially within the storage medium of a pressurized cylinder. The hydrogenation process often uses greater than 1 atmosphere of H2, usually conveyed from the cylinders and sometimes augmented by "booster pumps". Gaseous hydrogen is produced industrially from hydrocarbons by the process known as steam reforming.[9]
Hydrogen may, in specialised applications, also be extracted ("transferred") from "hydrogen-donors" in place of H2 gas. Hydrogen donors, which often serve as solvents include hydrazine, dihydronaphthalene, dihydroanthracene, isopropanol, and formic acid.[10] In organic synthesis, transfer hydrogenation is useful for the reduction of polar unsaturated substrates, such as ketones, aldehydes, and imines
BERGIUS PROCESS OF HYDRPGENATION OF COAL
The coal is finely ground and dried in a stream of hot gas. The dry product is mixed with heavy oil recycled from the process. Catalyst is typically added to the mixture. A number of catalysts have been developed over the years, including tungsten or molybdenum sulfides, tin or nickel oleate, and others. Alternatively, iron sulphides present in the coal may have sufficient catalytic activity for the process, which was the original Bergius process.
The mixture is pumped into a reactor. The reaction occurs at between 400 to 500 °C and 20 to 70 MPa hydrogen pressure. The reaction produces heavy oils, middle oils, gasoline, and gases
nC+(n+1)H2→CnH2n+2
The immediate product from the reactor must be stabilized by passing it over a conventional hydrotreating catalyst. The product stream is high in naphthenes and aromatics, low in paraffins and very low in olefins. The different fractions can be passed to further processing (cracking, reforming) to output synthetic fuel of desirable quality. If passed through a process such as Platforming, most of the naphthenes are converted to aromatics and the recovered hydrogen recycled to the process. The liquid product from Platforming will contain over 75% aromatics and has a RON of over 105.
Overall, about 97% of input carbon fed directly to the process can be converted into synthetic fuel. However, any carbon used in generating hydrogen will be lost as carbon dioxide, so reducing the overall carbon efficiency of the process.
There is a residue of unreactive tarry compounds mixed with ash from the coal and catalyst. To minimise the loss of carbon in the residue stream, it is necessary to have a low-ash feed. Typically the coal should be <10% ash by weight. The hydrogen required for the process can be also produced from coal or the residue by steam reforming. A typical hydrogen demand is ~8kg hydrogen per ton of dry, ash-free coal.
well note the date in right coloumn of the previos post.!!!!!!!!!!!!!!
waah bhai tu to chaa gaya bhai
didnt saw it earlier
now we will try it
KEEP UR GOOD WORK COMING[1]
@ bhargav
yaar Q5 mein fugicity hai ya fugacity [7]
vaise fugacity ka wiki pe kuch mila tha
Fugacity is a measure of a chemical potential in the form of 'adjusted pressure.' It reflects the tendency of a substance to prefer one phase (liquid, solid, or gas) over another, and can be literally defined as “the tendency to flee or escapeâ€. At a fixed temperature and pressure, a homogeneous substance will have a different fugacity for each phase. The phase with the lowest fugacity will be the most favorable, and will have the lowest Gibbs free energy. Fugacity has the same units as pressure (e.g., atm, psia, bars, etc.)
ab se integration ke saath saath din raat chemistry bhi padenge [1]
b555 please aur kuch post mat karna
let the erlier be finished
inorganic chem
Q3 (post184)
dekh bhai diamond has a better conductivity as compared to Cu
A diamond is a transparent crystal of tetrahedrally bonded carbon atoms (sp3) and crystallizes into the face centered cubic diamond lattice structure
Most notable is its extreme hardness, its high dispersion index, and extremely high thermal conductivity (900 – 2320 W/m K)
Whereas Cu is a metallic substance.It posses thermal conductivity due to the free valence electrons present in it as it is a paramagnetic substance.
Thermal conductivity : (300 K) 401 W·m−1·K−1
contd..........
Q2
In the boron trihalides, BX3, the length of the B-F bonds (1.30 Ã
) is shorter than would be expected for single bonds, and this shortness may indicate stronger B-X π-bonding in the fluoride. A facile explanation invokes the symmetry-allowed overlap of a p orbital on the boron atom with the in-phase combination of the three similarly oriented p orbitals on fluorine atoms.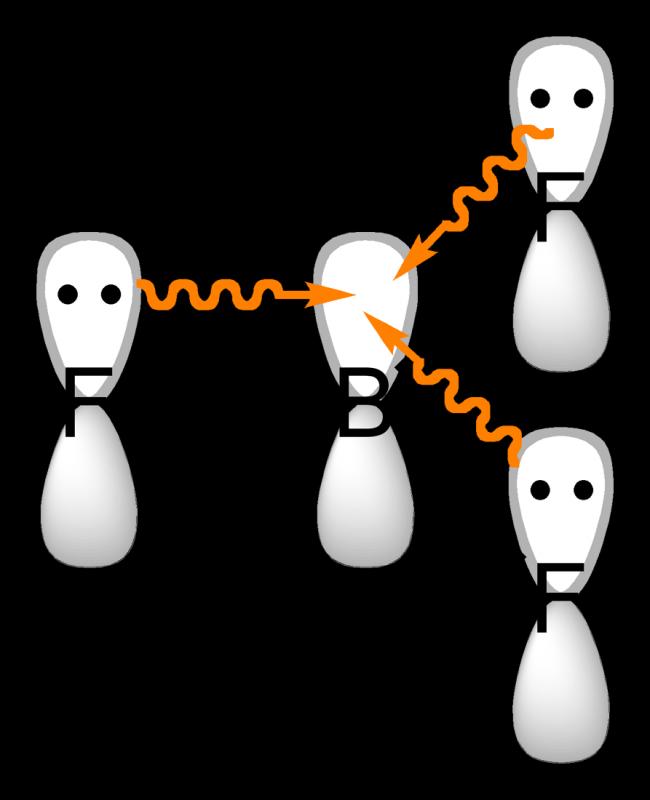
Q.6.
Statement 1: Alkenes can give electrophilic as well as nucleophilic addition reactions.
and
Statement 2: Addition of HCl to CH2 = CH - Cl will mainly produce CH3 - CHCl2.
1. Statement 1 is True, statement 2 is True; statement 2 is a correct explanation for statement 1.
2. Statement 1 is True, statement 2 is True; statement 2 is not a correct explanation for statement 1.
3. Statement 1 is True, statement 2 is False.
4. Statement 1 is False, statement 2 is True.
ANSWER TO THIS QUES (POST 180 ) WILL BE (B)PLEASE DO CONFIRM IT
AS S1 AND S2 BOTH R CORRECT BUT IMPLICATION FAILS!!!1
Q.7. KMnO4 and MnO2 react in alkaline medium (KOH) to give K2MnO4. What is n factor of K2MnO4 in the reaction?
1. 3
2. 2
3. 2/3
4. 3/2
iss mein n factor kya hai????????
Consider the possibility of the following reaction:
2-methyl-1-propanol
+
PBr3 & heat
mere khyal se iska answer1-bromo 2-methyl propane hona chayiye
b555 i think ur asking about fugacity in no.5..
ai x fi = ci
where fi is fugacity.
use dis useful information by the seniors
don't let dese threads die out
u should participate and discuss the questions present here to deepen ur knowledge!